Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A gas containing 80% CH4 and 20% He is sent through a quartz diffusion tube (see Figure P4.1.8) to recover the helium. Twenty percent
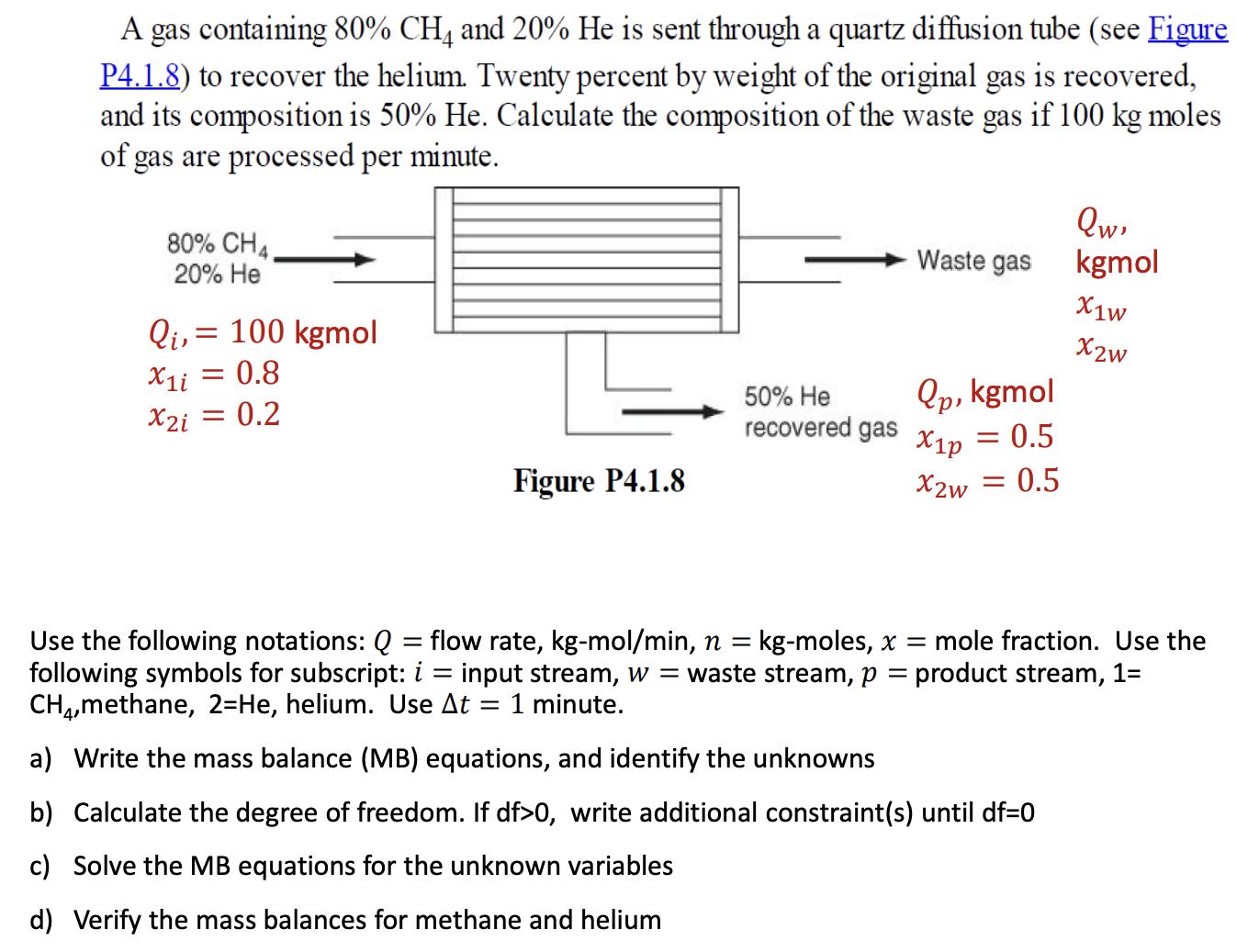
A gas containing 80% CH4 and 20% He is sent through a quartz diffusion tube (see Figure P4.1.8) to recover the helium. Twenty percent by weight of the original gas is recovered, and its composition is 50% He. Calculate the composition of the waste gas if 100 kg moles of gas are processed per minute. 80% CH4, 20% He Qi, = 100 kgmol X1i = 0.8 X2i = 0.2 Figure P4.1.8 - 50% He recovered gas Waste gas Qp, kgmol = 0.5 X1p X2w = 0.5 Qw, kgmol X1w X2w Use the following notations: Q = flow rate, kg-mol/min, n = kg-moles, x = mole fraction. Use the following symbols for subscript: i = input stream, w = waste stream, p = product stream, 1= CH4,methane, 2-He, helium. Use At 1 minute. a) Write the mass balance (MB) equations, and identify the unknowns b) Calculate the degree of freedom. If df>0, write additional constraint(s) until df=0 c) Solve the MB equations for the unknown variables d) Verify the mass balances for methane and helium
Step by Step Solution
★★★★★
3.47 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


