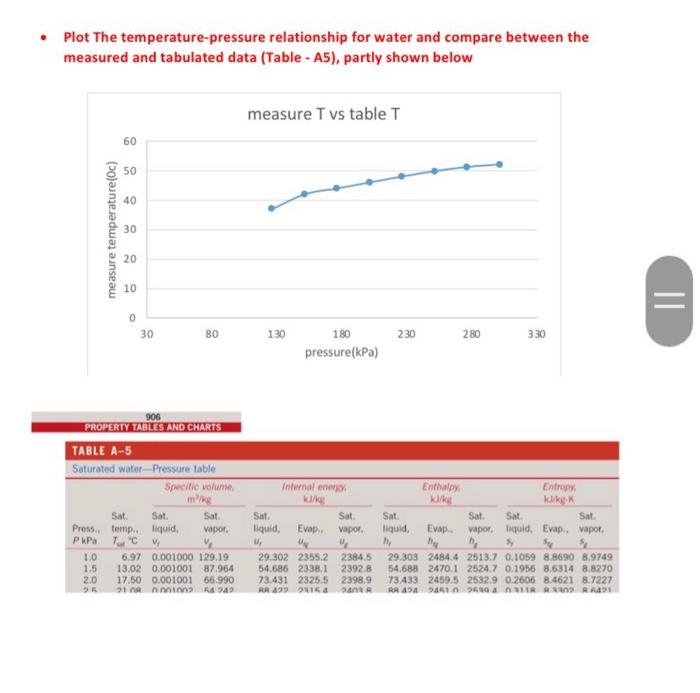
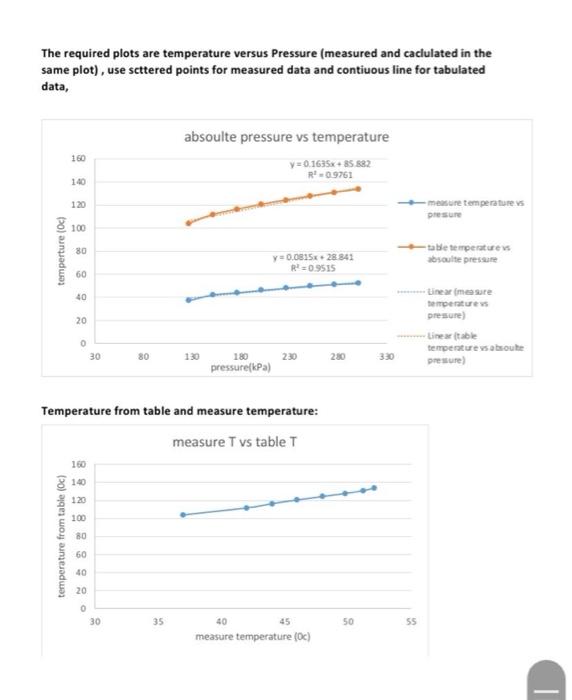
What is the best trend curve for the T-P relation and what is the correlation factor of this relation
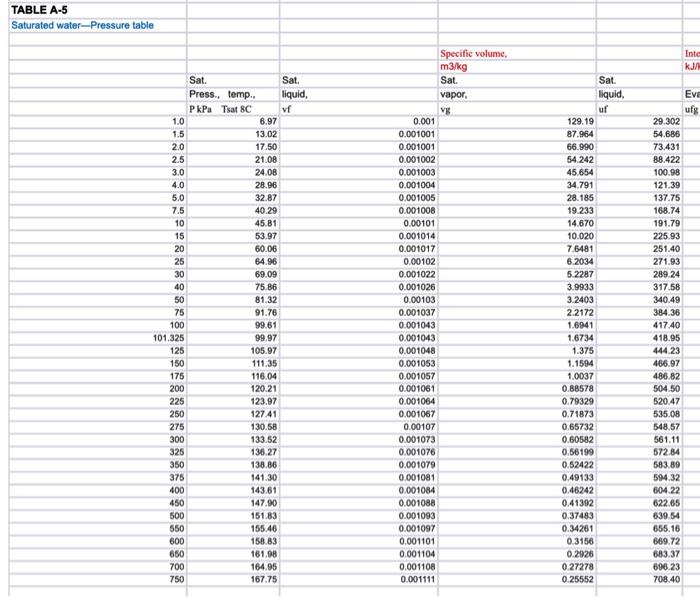
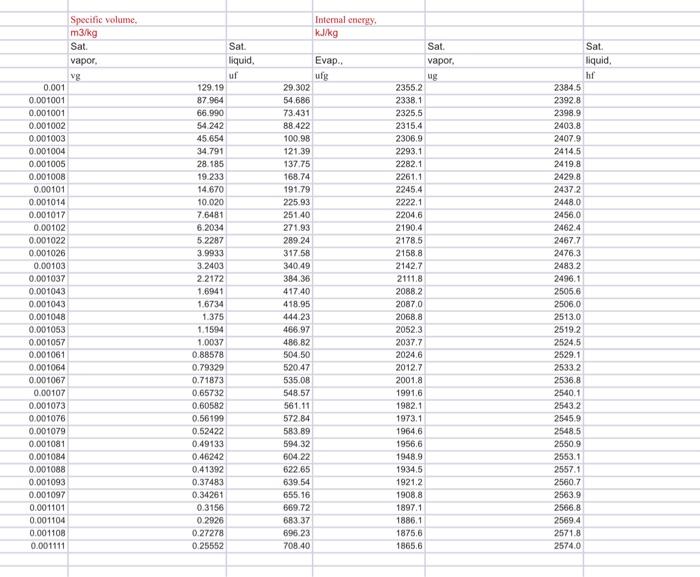
Plot The temperature-pressure relationship for water and compare between the measured and tabulated data Table - A5), partly shown below measure T vs table T 60 50 40 measure temperature (Oc) 30 20 10 0 30 80 130 230 280 330 180 pressure(kPa) 906 PROPERTY TABLES AND CHARTS TABLE A-5 Saturated water Pressure table Specific volume, Internal ener Enthly Entropy K Sat Sat Sat. vapor, liquid, Evap vapor Sat Sat liquid, Evap. Sat. vapor liquid Evap Sat Sat. Sat Press. temp. liquid, vapor, PAP . 1.0 6.97 0.001000 129.19 1.5 13.02 0.001001 87.964 2.0 17.50 0.00100166.990 91 ning 949 29.3022355.2 2384.5 54.686 2338.1 23928 73.431 2325.5 2398,9 RR 499016a BA 29.303 2484.4 2513.7 0.1059 8.8690 8.9749 54,688 2470.1 2524.7 0.1956 8.6314 8.8270 73.433 2459.5 25329 0.2606 8.4621 8.7227 RR 900 RR R The required plots are temperature versus Pressure (measured and caciulated in the same plot), use scttered points for measured data and contiuous line for tabulated data, 160 absoulte pressure vs temperature Y=0.1635x+35882 R! 09761 140 120 mere temperatures pressure 100 temperture (Oc) 80 y = 0.0815x28 841 R=D9515 table temperatures asculte pressure 60 40 Linear mesure temperatures pressure) 20 0 30 Lineartable temperatures about pressure) 80 130 230 280 330 180 pressure(kPa) Temperature from table and measure temperature: measure T vs table T 160 120 100 temperature from table (Oc) 80 60 40 20 0 30 35 40 45 50 55 measure temperature (0) TABLE A-5 Saturated water-Pressure table Inte 99999 1.0 1.5 2.0 2.5 3.0 4.0 5.0 7.5 10 15 20 25 30 40 50 75 100 101.325 125 150 175 200 225 Sat. Sat. Press, temp.. liquid, PkPa Tsat 8C vf 6.97 13.02 17.50 21.08 24.08 28.96 32.87 40.29 45.81 53.97 60.06 64.96 69.09 75.86 81.32 91.76 99.61 99.97 105.97 111.35 116.04 120.21 123.97 127.41 130.58 133.52 136.27 Specific volume, m3/kg Sat. vapor. vg 0.001 0.001001 0.001001 0.001002 0.001003 0.001004 0.001006 0.001008 0.00101 0.001014 0.001017 0.00102 0.001022 0.001026 0.00103 0.001037 0.001043 0.001043 0.001048 0.001053 0.001057 0.001061 0.001064 0.001067 0.00107 0.001073 0.001076 0.001079 0.001081 0.001084 0.001088 0.001093 0.001097 0.001101 0.001104 0.001108 0.001111 Sat. liquid, uf 129.19 87.964 66.990 54 242 45.654 34.791 28.185 19.233 14.670 10.020 7.6481 6.2034 5.2287 3.9933 3.2403 2.2172 1.6941 1.6734 1.375 1.1594 1.0037 0.88578 0.79329 0.71873 0,65732 0.60582 0.56199 0.52422 0.49133 0.46242 0.41392 0.37483 0.34261 0.3156 0.2926 0.27278 0.25552 EVE ufg 29.302 54.686 73.431 88.422 100.98 121.39 137.75 168.74 191.79 225.93 251.40 271.93 289.24 317.58 340.49 384.36 417.40 418.95 444 23 466.97 486.82 504.50 520.47 535.08 548,57 561.11 572.84 583.89 594.32 604.22 622.65 639.54 655.16 669.72 683.37 696.23 708.40 250 138.86 275 300 325 350 375 400 450 500 550 600 650 700 750 141.30 143.61 147.90 151.83 155.46 158.83 161.98 164.95 167.75 Internal energy kJ/kg Evap.. ufg Specific volume m3/kg Sat. vapor. vg 0.001 0.001001 0.001001 0.001002 0.001003 0.001004 0.001005 0.001008 0.00101 0.001014 0.001017 0.00102 0.001022 0.001026 0.00103 0.001037 0.001043 0.001043 0.001048 0.001053 0.001057 0.001061 0.001064 0.001067 0.00107 0.001073 0.001076 0.001079 0.001081 0.001084 0.001088 0.001093 0.001097 0.001101 0.001104 0.001108 0.001111 Sat liquid uf 129.19 87.964 66.990 54 242 45.654 34.791 28.185 19233 14.670 10.020 7.6481 6.2034 5.2287 3.9933 3.2403 2.2172 1.6941 1.6734 1.375 1.1594 1.0037 0.88578 0.79329 0.71873 0.65732 0.60582 0.56199 0.52422 0.49133 0.46242 0.41392 0.37483 0.34261 0.3156 0.2926 0.27278 0.25552 29.302 54.686 73.431 88.422 100.98 121.39 137.75 168.74 191.79 225.93 251.40 271.93 289.24 317.58 340.49 384.36 417.40 418.95 444.23 466.97 486.82 504.50 520.47 535.08 548.57 561.11 572.84 583.89 594.32 604.22 622.65 639 54 655.16 669.72 683.37 696.23 708.40 Sat. vapor. ug 2355.2 2338.1 2325.5 2315.4 2306.9 2293.1 2282.1 2261.1 2245.4 2222.1 2204.6 2190.4 2178.5 2158.8 2142.7 2111.8 2088.2 2087.0 2068.8 2052.3 2037.7 2024.6 2012.7 2001.8 1991.6 1982.1 1973.1 1964.6 1956.6 1948.9 1934.5 1921.2 1908.8 1897.1 1886.1 1875.6 1865.6 Sat liquid ht 2384,5 2392.8 2398.9 2403.8 2407.9 2414,5 2419.8 2429.8 24372 24480 2456.0 2462.4 2467.7 24763 2483.2 2496.1 25066 2506.0 2513.0 2519.2 2524.5 2529.1 25332 2536.8 2540.1 2543.2 2545.9 2548,5 2550 9 2553.1 2557.1 2560.7 2563.9 2566.8 2569.4 2571.8 2574.0