Question
What is the freezing point of 22.1 g NaCl in 114 mL of water. Round your answer to 3 significant digits. Freezing point: Part
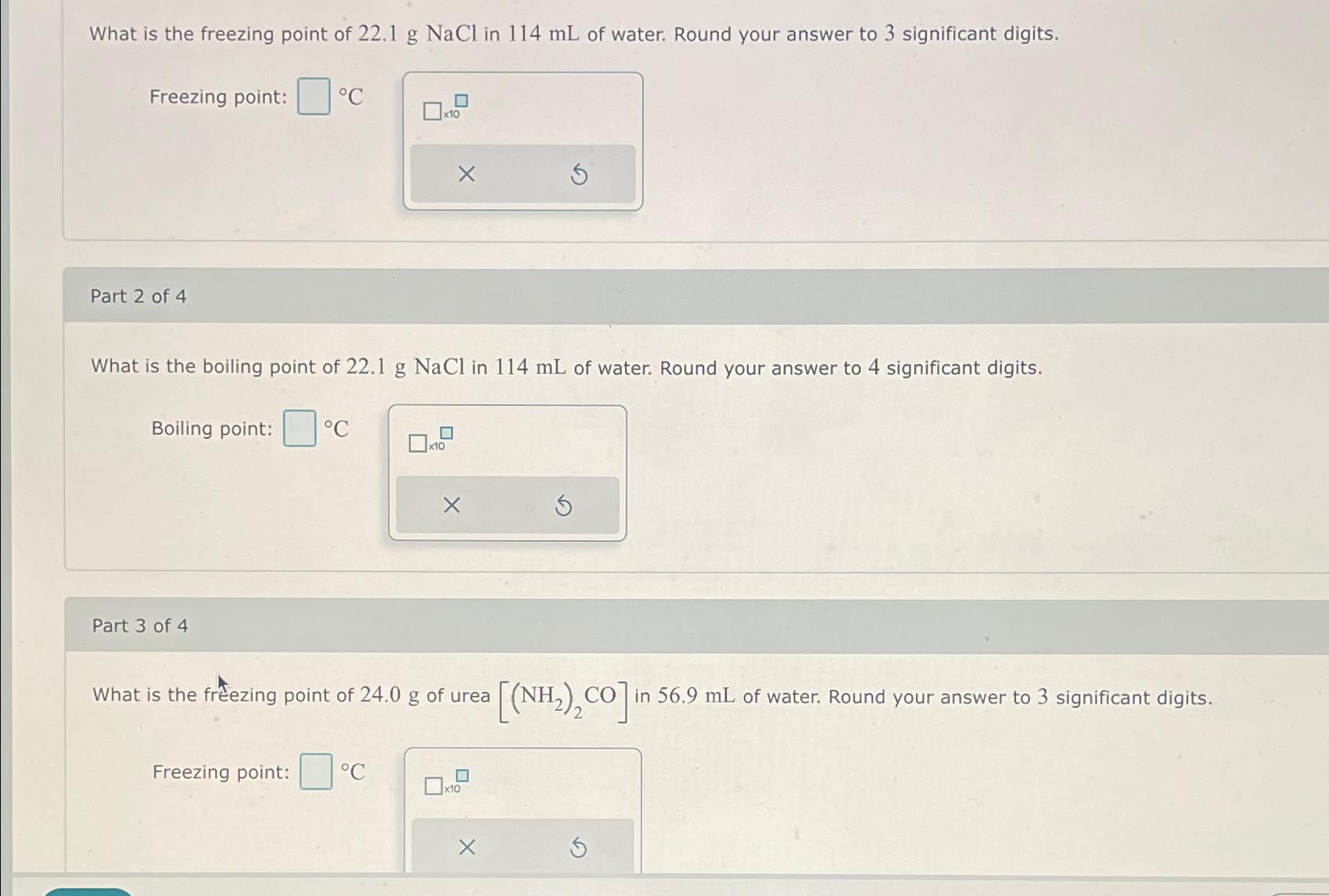
What is the freezing point of 22.1 g NaCl in 114 mL of water. Round your answer to 3 significant digits. Freezing point: Part 2 of 4 What is the boiling point of 22.1 g NaCl in 114 mL of water. Round your answer to 4 significant digits. Boiling point: C Part 3 of 4 x10 Freezing point: x10 X What is the freezing point of 24.0 g of urea [(NH,),CO]in in 56.9 mL of water. Round your answer to 3 significant digits. x10 X
Step by Step Solution
3.44 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
Solutions Step 1 To calculate the freezing point depression and boiling point elevation you can use ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Introductory Chemistry version 1.0
Authors: David W. Ball
1st edition
1453327657, 978-1453327654
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



