Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What is the main reason why benzoin has a higher melting point than benzil? The dominant intermolecular forces in benzoin are dipole-dipole interactions. The
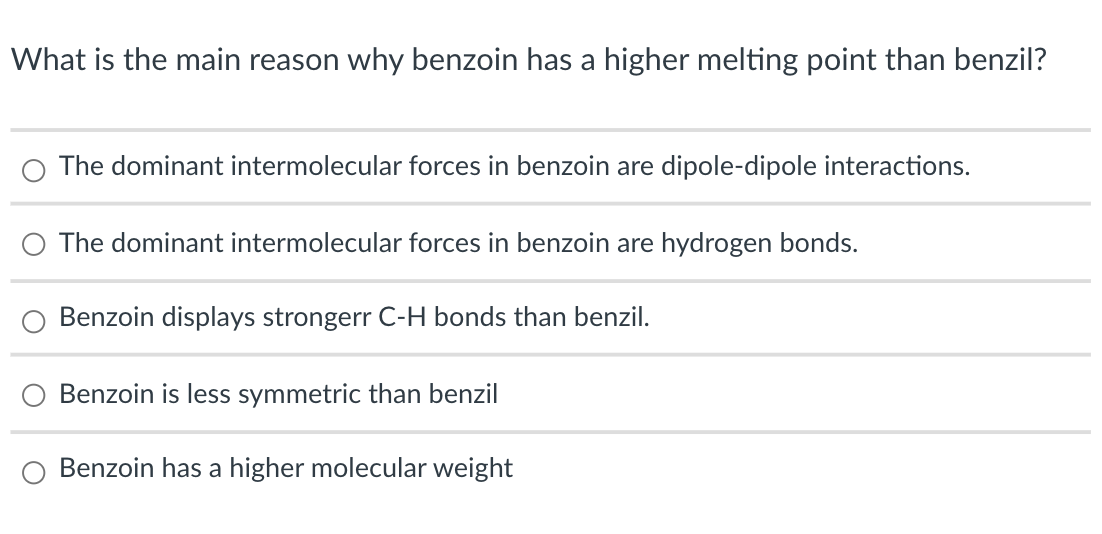
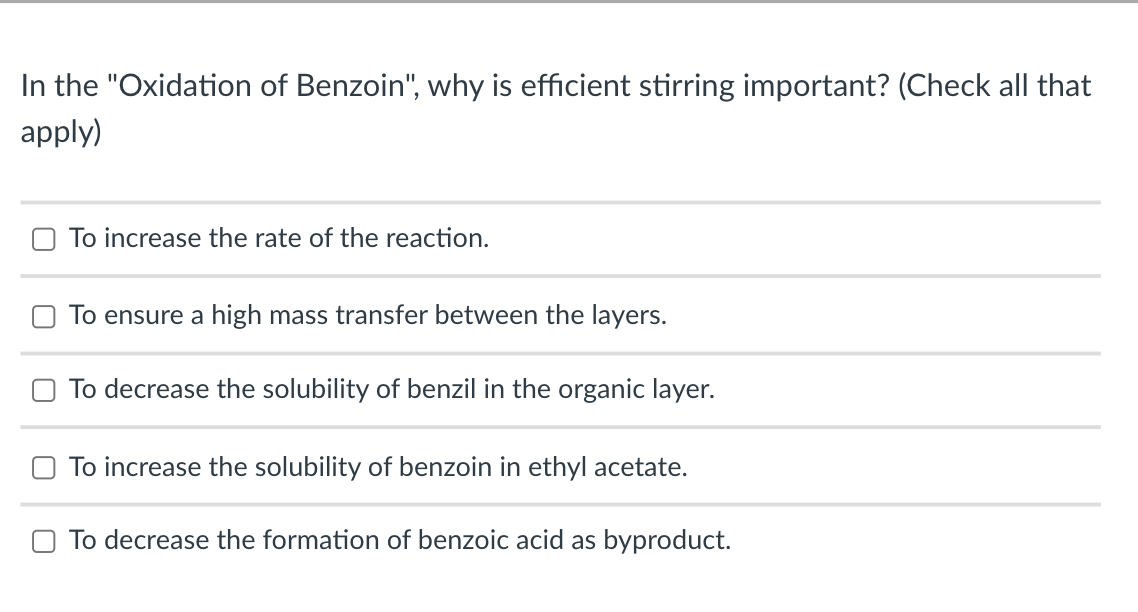
What is the main reason why benzoin has a higher melting point than benzil? The dominant intermolecular forces in benzoin are dipole-dipole interactions. The dominant intermolecular forces in benzoin are hydrogen bonds. Benzoin displays strongerr C-H bonds than benzil. Benzoin is less symmetric than benzil Benzoin has a higher molecular weight In the "Oxidation of Benzoin", why is efficient stirring important? (Check all that apply) To increase the rate of the reaction. To ensure a high mass transfer between the layers. To decrease the solubility of benzil in the organic layer. To increase the solubility of benzoin in ethyl acetate. To decrease the formation of benzoic acid as byproduct.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


