Question
What is the percent yield of a reaction that produces 12.5 g of the gas Freon CFCl from 32.9 g of CCl4 and excess
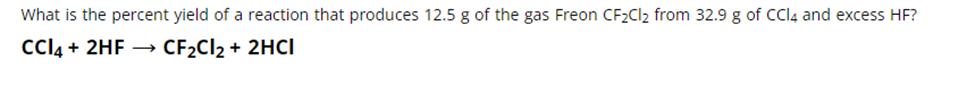
What is the percent yield of a reaction that produces 12.5 g of the gas Freon CFCl from 32.9 g of CCl4 and excess HF? CCI4 + 2HF CFCl2 + 2HCI
Step by Step Solution
3.34 Rating (148 Votes )
There are 3 Steps involved in it
Step: 1
The percent yield of the reaction is 9803 Heres how we can calculate it Calcul...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemistry A Molecular Approach
Authors: Nivaldo Tro
5th Edition
0134874374, 978-0134874371
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



