Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What is the pH of a solution containing 0.010MH3PO4 and 0.10MNaH2PO4 ? The pKa values for phosphoric acid are pKK1=2.15,pKK2=6.82, and pKK3=12.38. Note: Your answer
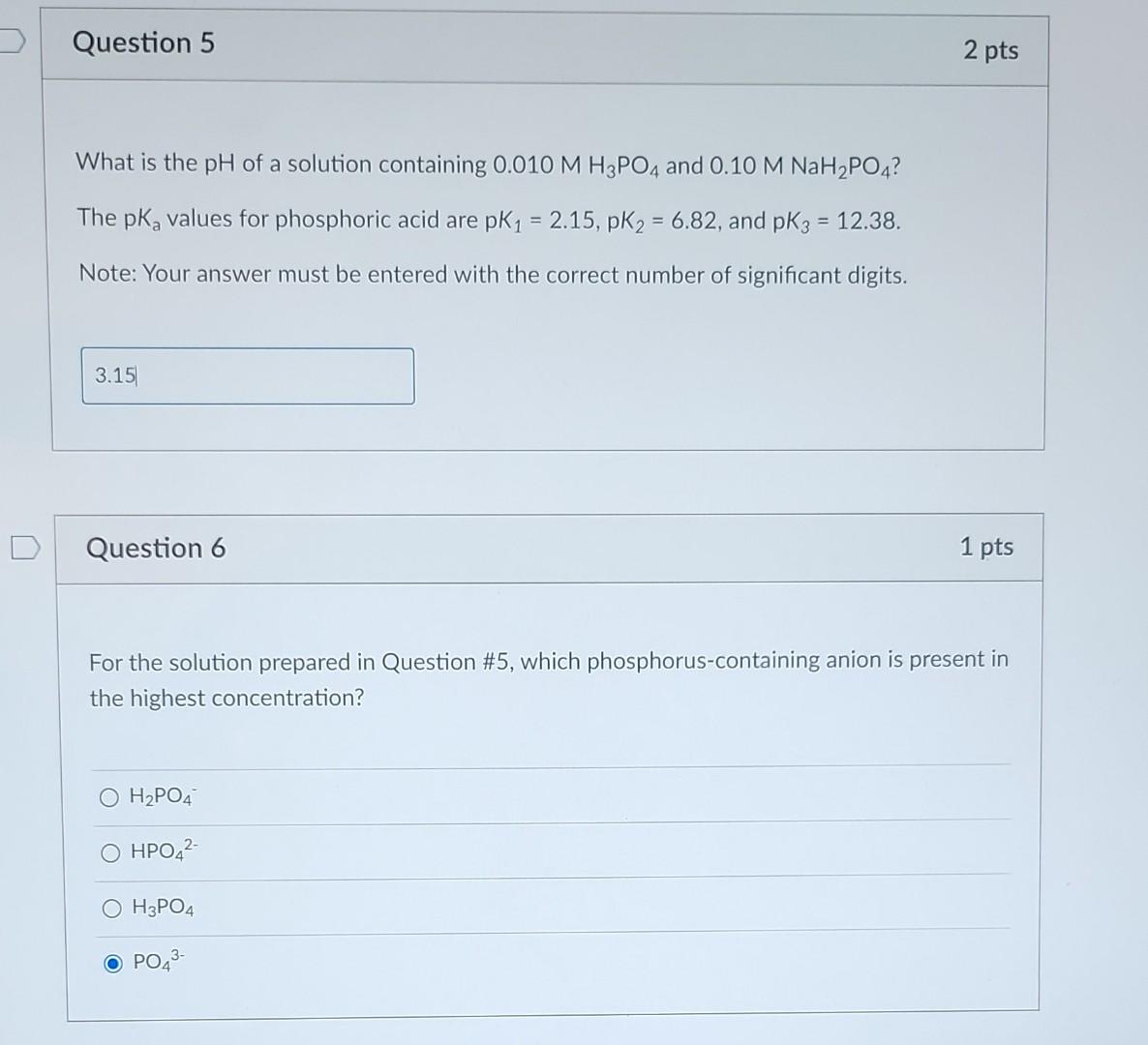

What is the pH of a solution containing 0.010MH3PO4 and 0.10MNaH2PO4 ? The pKa values for phosphoric acid are pKK1=2.15,pKK2=6.82, and pKK3=12.38. Note: Your answer must be entered with the correct number of significant digits. Question 6 1 pts For the solution prepared in Question \#5, which phosphorus-containing anion is present in the highest concentration? H2PO4 HPO42 H3PO4 PO43 The dominant anion present in the solution from Question \#5 has which of the following properties (select all that apply)? A negative charge Can participate in Van der Waals interactions Is an H-bond donor Is an H-bond acceptor A positive charge
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


