Question
When the ambient temperature is 300K, the cold energy of Qo=1000kJ is extracted from the cold source at 250K. What is the cold exergy
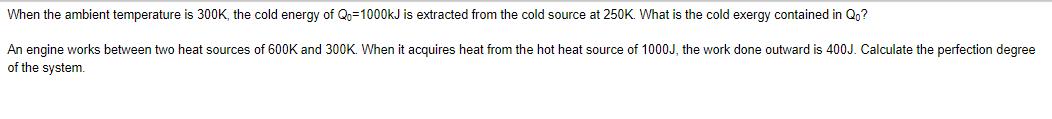
When the ambient temperature is 300K, the cold energy of Qo=1000kJ is extracted from the cold source at 250K. What is the cold exergy contained in Qo? An engine works between two heat sources of 600K and 300K. When it acquires heat from the hot heat source of 1000J, the work done outward is 400J. Calculate the perfection degree of the system.
Step by Step Solution
3.42 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
To determine the cold exergy of Qo we use the following formula Ec Qo1 TcTh where Ec is the cold exe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Income Tax Fundamentals 2013
Authors: Gerald E. Whittenburg, Martha Altus Buller, Steven L Gill
31st Edition
1111972516, 978-1285586618, 1285586611, 978-1285613109, 978-1111972516
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



