Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What is the solution? Given Required Solution 100cm HCl 0.200M 500mm HNO 0200M 300cm NaOH 0.100M NO M 100cm* of 0.200M HCI + 500min of
What is the solution? 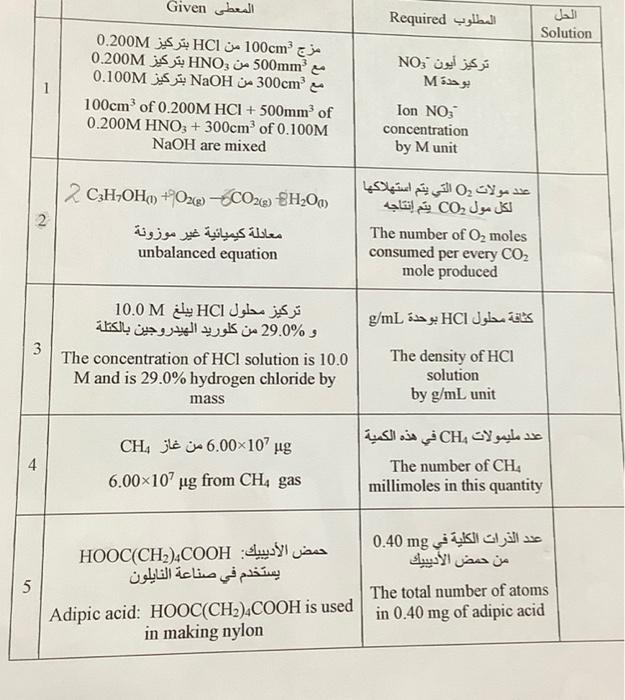
Given Required Solution 100cm HCl 0.200M 500mm HNO 0200M 300cm NaOH 0.100M NO M 100cm* of 0.200M HCI + 500min of 0200M HNO3 + 300cm3 of 0.100M NaOH are mixed Ion NO; concentration by Munit (SH200 (CH,OH +O2e) - CO 0 CO unbalanced equation The number of Oz moles consumed per every CO2 mole produced HCl M 10.0 %29.0 HCl g/mL 3 The concentration of HCl solution is 10.0 Mand is 29.0% hydrogen chloride by mass The density of HCI solution by g/mL unit CH 4 6.00x107 Mg CH g from CH, gas 6.00x107 The number of CH. millimoles in this quantity : HOOC(CH),COOH mg 0.40 5 The total number of atoms Adipic acid: HOOC(CH2),COOH is used in 0.40 mg of adipic acid in making nylon 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


