Question: What is the table of reagents; include name, structure, molecular weight, melting and boiling point, and density? What are the main reactions? What are the

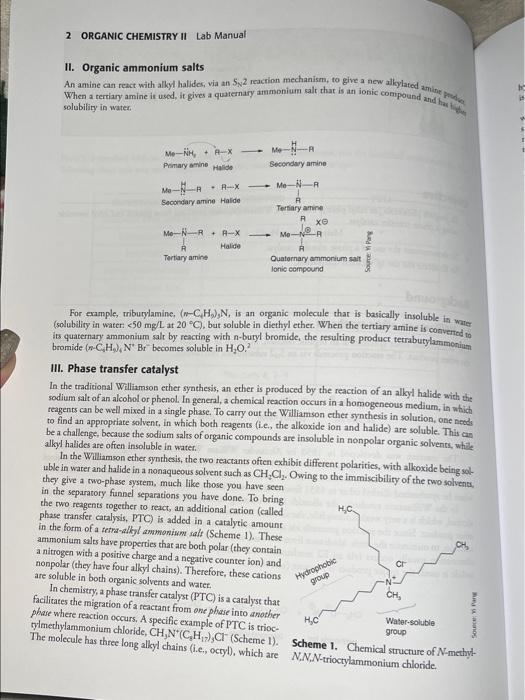
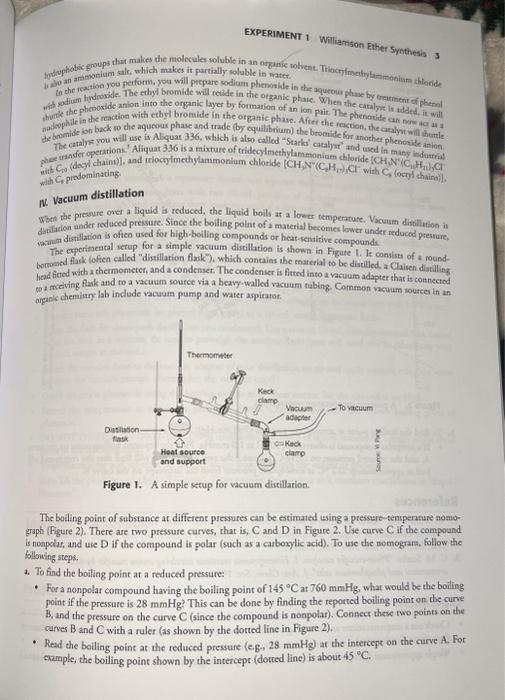


To pacpare phesceole (or ethyl phenyl ether) by a Williamson ether synthetis from phenol and ethyl beomide asing a phase transfer catalyyt. MainReaction:Ph-O+EtBrPhQEt - To demonstrate the use of a phase transfer catalyst. - To Hlastrate the use of vacuum distillation. Background I. Williamson ether synthesis The Williamson cther synthesis is an organic reaction, in which an alloside reaces with an alky| halide to form the elher prodact. The reaction procesds via an SN2 abititution mochunism: The reaccion was developed in 1850 by Alexander Willians Walliamson (an English chemist), and as importane in the history of organic chemistry. In 1544 , an organic compound was prepared by heating echanol with sulfarie acid. The cocrect formuls of the compound was firse ertablihhed to be diethyl ether by Williamsoat, when he synthesized dicthyl erher in 1851 from sodium ethoxide and ethyl chloride. In the Walliamson synthesis, the alloxide ion is a strong nucicophile as well as a powerful base. Unlike the alcohol, which is a very weak nucleophile, the alloxide ion reacts with primary halides to form the cosresponding etler product. Since the reaction procecds via an SN2 mechanism, a primary alkyl halide is typically uscd in the reaction, to minimine the steric hindrance from the back-side atrack. If the alkyl halide is not primary, significanc elimination usually occurs. (via an F2 mechanism). For example, when a secondary halide asch as isopropyl Sodide is used, the major product is propene (not ether). II. Organic ammonium salts An amine can react with alkyl halides, via an 5N2 reacrion mechanism, to give a new allylated amine pey When a terriary amine it used, it gives a quarernary ammonium alt that is an ionic conpound and has hys. solubility in watex For example, rriburylamine, (nC4H2)3N, is an organic molecule that is basically insoluble is wate (solubility in waten 60mg/L at 20C ), but soluble in diethyl ether. When the tertiary amine is converned to its quaternary ammonium salt by reacring with n-butyl bromide, the resulting product tetrabutylammonilum bromide (nC4H3)4N Br becomes soluble in H2O? III. Phase transfer catalyst In the traditional Williamson ether synthesis, an ether is produced by the reaction of an alkyl halide with the sodium salt of an alcohol or phenol. In genenal, a chemical reaction occurs in a homogeneous medium, in which reagents can be well mixed in 2 single phase. To carry out the Williamson ether synthesis in solution, one necds. to find an appropriate solvent, in which both reagents (i.e., the alkoxide ion and halide) are soluble. This cas be a challenge, because the sodium salts of organic compounds are insoluble in nonpolar onganic solvents, while, alkyl halides are often insoluble in water. In the Williamson echer synthesis, the two reactants often exhibit different polarities, with alkoxide being soluble in water and halide in a nonaqueous solvent such as CH2Cl2. Owing to the immiscibility of the two solvent, they give a two-phase system, much like those you have seen. in the separatory funnel separations you have done. To bring the two reagents togecher to react, an additional cation (called phase transfer catalysis, PTC) is added in a catalytic amount in the form of a tetri-allyl ammonium salt (Scheme 1). These ammonium salts have properties that are both polar (they concain a nitrogen with a positive charge and a negative counter ion) and nonpolar (chey have four alkyl chains). Therefore, these cations are soluble in both organic solvents and water, In chemistry, a phase transfer catalyst (PTC) is a catalyst that facilitates the migration of a reactant from one phase into another phase where reaction occurs. A specific example of PTC is trioctylmethylammonium chloride, CH3N+(C8H17)3Cl(Scheme 1). sueme 1. Chemical structure of N-methylThe molecule has three long alkyl chains (i.e,, octyl), which are N,N,N-trioctylammonium chloride. EXPERIMENT 1 Willanson Ether Synthesis 3 with yadium thydroside the anion into the organic layer by formation of an ion poit. The phenerite is addes, is will with C20 ionjedemaine IV. Vacuum distillation Whin the presure over a liquid is reduced, the liquid boils at a lower temperature. Vacuim distillution in dialation ander reduced preasure. Since the boiling point of a material beconies lower under reducod presture. The coperimental serup for a simple vacuum dissillation is shown in Figute 1. It consise of a roundbotroned flask (ofien called "distillation flakk"). which contaias the marerial to be distilled, a Claisen datilling liad firted with a thermometer, and a condenser. The condenser is firted into a vacuum adapter that is coenectred ea mceiving flak and to a vacuam soufce via a heavy-walled vacuum tabing. Common vacuum source in an Figure I. A simple sttup for vacuum dirtillation. The boiling point of substance ar difterent pressures can be estimated using a pressere-tensperature nomograph (Figure 2). There are rwo pressure curves, rhat is, C and D in Figure 2. Use curve C if the compoand is noapolar, and uie D if the compound is polar (such as a carboxylic acid). To use the nomogrars, follow the Gollowing steps. a. To find the boiling point at a reduced pressure: - For a nonpolar compound having the boiling point of 145C at 760mmHg, what would be the boiling point if the pressure is 28mmHg. This can be done by finding the reported boiling point on the curve B, and the pressure on the curve C (since the compound is nonpolar). Connecr these two poins on the curves B and C with a ruler (as shown by the dorted line in Figure 2). - Read the bolling point at the reduced pressure (e.g28mmHg at the intercept on the curve A. For cample, the boiling point shown by the intercept (dorred line) is about 45C. 4. ORCANIC CHEMastat if Iab Mraut References Lide, Fat CivC Hins, New York, 200his Experiment flet trum Stock rrom), - To the fluk add 25ml. of methylene ctioride and AmL. of etryt tromide. - Ade 1 mL of micaprylmethyiammonism chloride uslng a pippene. - Eevip the fiats widy a warer-cooled condenser and a heating mantle. - See wir bexing mantle controller ta 4. - Wiab good reiering retlux the miature for a period of 3b. This refluxing step can be split up over a two lab periods. Before mantitg so unasemble the triles plawart actiseg, lower the heating mantle, and waic for the device to cool. Do sot brok the glaimente? - Farad the remaining aqaeous layes with rwo. 10-mL- porvions of meeblese chloride. Srpurar the kewe orginic layer exch cime and combine there wahhings with the first otganic layer. - Claas out the separatory funnel (disard the aqueous layer). - Recuen the combined layers to the separatory funnel and wash with 15ml of 2M sodiam hradroulde. Collect. the lower orgalic layer, and discard the upper aqueous layer. - Renair the onganic layer to the separatory fuanel and wash it with 15mL of araratod voclium chloeder solo: don. Callest the lower organic layer. - Dry de erganic Lyer with ealcium chlonide pelles (ahout 0.5g and tranter the organic liequid r a Faneur pipetie to a small Erlensecyer flaskand evaporate the wolvent thoroaghly on the sterm buth wian 100 atram. - Transfer the crude ploenetole (or phenyl ethyl ether) to a 10-ml roand-botromnd flak - Bquip the thask for shorr-path ditrillationi (no condenser). Use a socond 10-ml sound boctomed fakk cooles in as ies bath as a receiver. Be sure so prewcigh the recciver flaild (you will neod this in calculating yout prodact weight at the end.) - Auadh the vacuam hose to the vacuans adapter of your distiliation ses up and apply vacuum uilng the water aspirats (or vacuam pump), (Remember the vacuum trapl) - Distll the crude phenecole using the heating mantle as the heat source. Set the consoller on High. - Reword the temperature at which the phenetole distills. Compare this with the lonown boiling point of phenecole. - DO NOT DISTILL. TO DRYNESS. Ditill until a small amount of liquid remaina or a fopey white doed forms is the distilling flask- - Weigh she receiver and sabtrace the weight of the flask to obtain the weighe of pheneeole. - Sow TA the product and obcain TA hnirial. Then transfer the prodoct so a concainer that hhelas as "Exp 71 Phenetole: Laboratory Notebook Lhe the format provided in this handout packer. Be sure to provide a buanced oqration for the syatheria of puenceole. Product - The product will include the phenetole product \{colorless, water-insoluble liquid, bp 171-173 9C and indicare the amounc. - The product should also include a calcularion of theoretical and percent yields based on the limiring ragens. and erimation of the pressure of vacuum pump (use the graph in Figure 2). To pacpare phesceole (or ethyl phenyl ether) by a Williamson ether synthetis from phenol and ethyl beomide asing a phase transfer catalyyt. MainReaction:Ph-O+EtBrPhQEt - To demonstrate the use of a phase transfer catalyst. - To Hlastrate the use of vacuum distillation. Background I. Williamson ether synthesis The Williamson cther synthesis is an organic reaction, in which an alloside reaces with an alky| halide to form the elher prodact. The reaction procesds via an SN2 abititution mochunism: The reaccion was developed in 1850 by Alexander Willians Walliamson (an English chemist), and as importane in the history of organic chemistry. In 1544 , an organic compound was prepared by heating echanol with sulfarie acid. The cocrect formuls of the compound was firse ertablihhed to be diethyl ether by Williamsoat, when he synthesized dicthyl erher in 1851 from sodium ethoxide and ethyl chloride. In the Walliamson synthesis, the alloxide ion is a strong nucicophile as well as a powerful base. Unlike the alcohol, which is a very weak nucleophile, the alloxide ion reacts with primary halides to form the cosresponding etler product. Since the reaction procecds via an SN2 mechanism, a primary alkyl halide is typically uscd in the reaction, to minimine the steric hindrance from the back-side atrack. If the alkyl halide is not primary, significanc elimination usually occurs. (via an F2 mechanism). For example, when a secondary halide asch as isopropyl Sodide is used, the major product is propene (not ether). II. Organic ammonium salts An amine can react with alkyl halides, via an 5N2 reacrion mechanism, to give a new allylated amine pey When a terriary amine it used, it gives a quarernary ammonium alt that is an ionic conpound and has hys. solubility in watex For example, rriburylamine, (nC4H2)3N, is an organic molecule that is basically insoluble is wate (solubility in waten 60mg/L at 20C ), but soluble in diethyl ether. When the tertiary amine is converned to its quaternary ammonium salt by reacring with n-butyl bromide, the resulting product tetrabutylammonilum bromide (nC4H3)4N Br becomes soluble in H2O? III. Phase transfer catalyst In the traditional Williamson ether synthesis, an ether is produced by the reaction of an alkyl halide with the sodium salt of an alcohol or phenol. In genenal, a chemical reaction occurs in a homogeneous medium, in which reagents can be well mixed in 2 single phase. To carry out the Williamson ether synthesis in solution, one necds. to find an appropriate solvent, in which both reagents (i.e., the alkoxide ion and halide) are soluble. This cas be a challenge, because the sodium salts of organic compounds are insoluble in nonpolar onganic solvents, while, alkyl halides are often insoluble in water. In the Williamson echer synthesis, the two reactants often exhibit different polarities, with alkoxide being soluble in water and halide in a nonaqueous solvent such as CH2Cl2. Owing to the immiscibility of the two solvent, they give a two-phase system, much like those you have seen. in the separatory funnel separations you have done. To bring the two reagents togecher to react, an additional cation (called phase transfer catalysis, PTC) is added in a catalytic amount in the form of a tetri-allyl ammonium salt (Scheme 1). These ammonium salts have properties that are both polar (they concain a nitrogen with a positive charge and a negative counter ion) and nonpolar (chey have four alkyl chains). Therefore, these cations are soluble in both organic solvents and water, In chemistry, a phase transfer catalyst (PTC) is a catalyst that facilitates the migration of a reactant from one phase into another phase where reaction occurs. A specific example of PTC is trioctylmethylammonium chloride, CH3N+(C8H17)3Cl(Scheme 1). sueme 1. Chemical structure of N-methylThe molecule has three long alkyl chains (i.e,, octyl), which are N,N,N-trioctylammonium chloride. EXPERIMENT 1 Willanson Ether Synthesis 3 with yadium thydroside the anion into the organic layer by formation of an ion poit. The phenerite is addes, is will with C20 ionjedemaine IV. Vacuum distillation Whin the presure over a liquid is reduced, the liquid boils at a lower temperature. Vacuim distillution in dialation ander reduced preasure. Since the boiling point of a material beconies lower under reducod presture. The coperimental serup for a simple vacuum dissillation is shown in Figute 1. It consise of a roundbotroned flask (ofien called "distillation flakk"). which contaias the marerial to be distilled, a Claisen datilling liad firted with a thermometer, and a condenser. The condenser is firted into a vacuum adapter that is coenectred ea mceiving flak and to a vacuam soufce via a heavy-walled vacuum tabing. Common vacuum source in an Figure I. A simple sttup for vacuum dirtillation. The boiling point of substance ar difterent pressures can be estimated using a pressere-tensperature nomograph (Figure 2). There are rwo pressure curves, rhat is, C and D in Figure 2. Use curve C if the compoand is noapolar, and uie D if the compound is polar (such as a carboxylic acid). To use the nomogrars, follow the Gollowing steps. a. To find the boiling point at a reduced pressure: - For a nonpolar compound having the boiling point of 145C at 760mmHg, what would be the boiling point if the pressure is 28mmHg. This can be done by finding the reported boiling point on the curve B, and the pressure on the curve C (since the compound is nonpolar). Connecr these two poins on the curves B and C with a ruler (as shown by the dorted line in Figure 2). - Read the bolling point at the reduced pressure (e.g28mmHg at the intercept on the curve A. For cample, the boiling point shown by the intercept (dorred line) is about 45C. 4. ORCANIC CHEMastat if Iab Mraut References Lide, Fat CivC Hins, New York, 200his Experiment flet trum Stock rrom), - To the fluk add 25ml. of methylene ctioride and AmL. of etryt tromide. - Ade 1 mL of micaprylmethyiammonism chloride uslng a pippene. - Eevip the fiats widy a warer-cooled condenser and a heating mantle. - See wir bexing mantle controller ta 4. - Wiab good reiering retlux the miature for a period of 3b. This refluxing step can be split up over a two lab periods. Before mantitg so unasemble the triles plawart actiseg, lower the heating mantle, and waic for the device to cool. Do sot brok the glaimente? - Farad the remaining aqaeous layes with rwo. 10-mL- porvions of meeblese chloride. Srpurar the kewe orginic layer exch cime and combine there wahhings with the first otganic layer. - Claas out the separatory funnel (disard the aqueous layer). - Recuen the combined layers to the separatory funnel and wash with 15ml of 2M sodiam hradroulde. Collect. the lower orgalic layer, and discard the upper aqueous layer. - Renair the onganic layer to the separatory fuanel and wash it with 15mL of araratod voclium chloeder solo: don. Callest the lower organic layer. - Dry de erganic Lyer with ealcium chlonide pelles (ahout 0.5g and tranter the organic liequid r a Faneur pipetie to a small Erlensecyer flaskand evaporate the wolvent thoroaghly on the sterm buth wian 100 atram. - Transfer the crude ploenetole (or phenyl ethyl ether) to a 10-ml roand-botromnd flak - Bquip the thask for shorr-path ditrillationi (no condenser). Use a socond 10-ml sound boctomed fakk cooles in as ies bath as a receiver. Be sure so prewcigh the recciver flaild (you will neod this in calculating yout prodact weight at the end.) - Auadh the vacuam hose to the vacuans adapter of your distiliation ses up and apply vacuum uilng the water aspirats (or vacuam pump), (Remember the vacuum trapl) - Distll the crude phenecole using the heating mantle as the heat source. Set the consoller on High. - Reword the temperature at which the phenetole distills. Compare this with the lonown boiling point of phenecole. - DO NOT DISTILL. TO DRYNESS. Ditill until a small amount of liquid remaina or a fopey white doed forms is the distilling flask- - Weigh she receiver and sabtrace the weight of the flask to obtain the weighe of pheneeole. - Sow TA the product and obcain TA hnirial. Then transfer the prodoct so a concainer that hhelas as "Exp 71 Phenetole: Laboratory Notebook Lhe the format provided in this handout packer. Be sure to provide a buanced oqration for the syatheria of puenceole. Product - The product will include the phenetole product \{colorless, water-insoluble liquid, bp 171-173 9C and indicare the amounc. - The product should also include a calcularion of theoretical and percent yields based on the limiring ragens. and erimation of the pressure of vacuum pump (use the graph in Figure 2)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


