Answered step by step
Verified Expert Solution
Question
1 Approved Answer
what is the theoretical yeild if 48mL of vegetable oil were used. 12 mL of methanol. And thr calculated molecular weight of product of biodiesel
what is the theoretical yeild if 48mL of vegetable oil were used. 12 mL of methanol. And thr calculated molecular weight of product of biodiesel was calculated to be 296.12g/mol 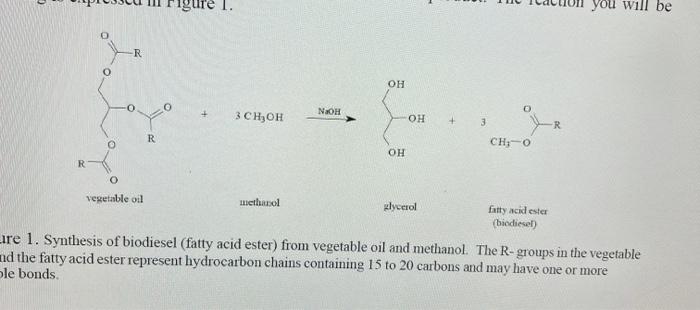
igure 1. you will be R OH O 3 CH, OH OH + 3 R R CHO OH R 0 vegetable oil methanol glycerol fatty acid ester biodiesel Lire 1. Synthesis of biodiesel (fatty acid ester) from vegetable oil and methanol. The R- groups in the vegetable nd the fatty acid ester represent hydrocarbon chains containing 15 to 20 carbons and may have one or more le bonds 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


