Question
What is the volume of water at 23 C with a density of 1.00 g/mL must be mixed with 180 mL (about 6 oz)
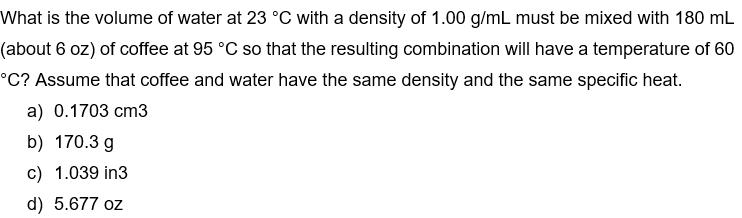
What is the volume of water at 23 C with a density of 1.00 g/mL must be mixed with 180 mL (about 6 oz) of coffee at 95 C so that the resulting combination will have a temperature of 60 C? Assume that coffee and water have the same density and the same specific heat. a) 0.1703 cm3 b) 170.3 g c) 1.039 in3 d) 5.677 oz
Step by Step Solution
There are 3 Steps involved in it
Step: 1
First we find the mass of water We know that 1g 1ml therefore 180ml 180g Heat ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elementary Principles of Chemical Processes
Authors: Richard M. Felder, Ronald W. Rousseau
3rd Edition
978-0471687573, 9788126515820, 978-0-471-4152, 0471720631, 047168757X, 8126515821, 978-0471720638
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



