Question: A fluid of unknown density is used in two manometers-one sealed-end, the other across an orifice in a water pipeline. The readings shown here are
A fluid of unknown density is used in two manometers-one sealed-end, the other across an orifice in a water pipeline. The readings shown here are obtained on a day when barometric pressure is 756mm Hg. What is the pressure drop (mm Hg) from point (a) to point (b)?
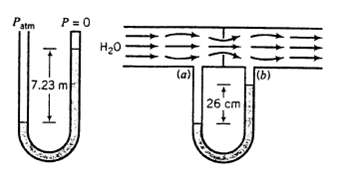
Patm P=0 7.23 m U HO #3 FEE (a) (b) T 26 cm + CA
Step by Step Solution
3.27 Rating (153 Votes )
There are 3 Steps involved in it
P atm PPg723 m P 723 g Pf P P P Pg2... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (88).pdf
180 KBs PDF File
13-E-C-E-C-P (88).docx
120 KBs Word File


