Answered step by step
Verified Expert Solution
Question
1 Approved Answer
What mass of ammonium nitrate at 65deg C must be added to a 211.0mL sample of water at 65deg C in order to cool the
What mass of ammonium nitrate at
65\\\\deg Cmust be added to a
211.0mLsample of\ water at
65\\\\deg Cin order to cool the solution to
58.0\\\\deg C. The heat capacity of the\ solution is
4.18(J)/(g)*^(0)C. The density of water is
1(g)/(m)L.\
NH_(4)NO_(3)(aq)->NH_(4)^(+)(aq)+NO_(3)^(-)(aq),\\\\Delta H_(rxn)=+25.69k(J)/(m)ol\ (A)
20.32g\ (B)
21.17g\ (C)
23.50g\ (D)
19.69g\ (E)
18.42g 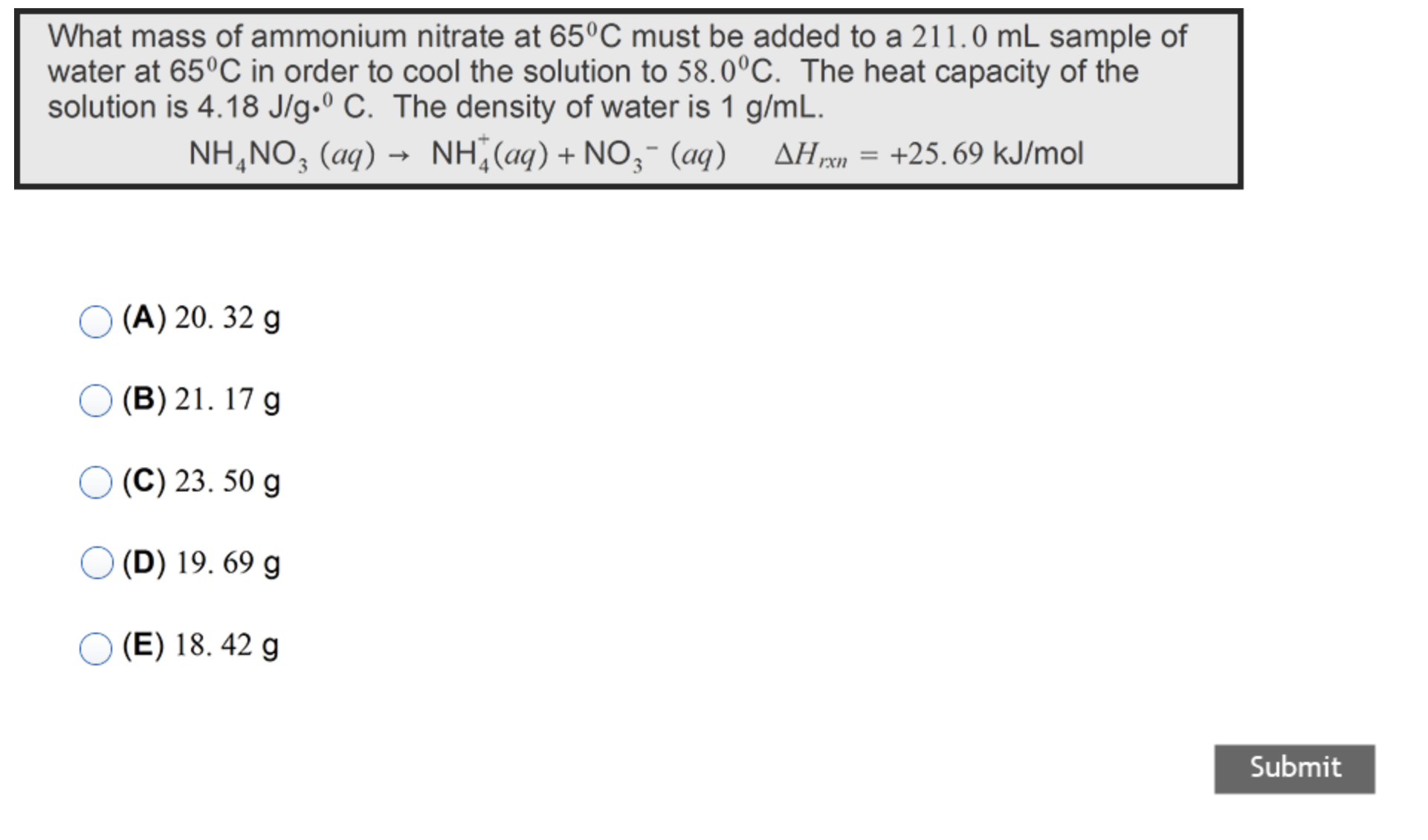
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


