Answered step by step
Verified Expert Solution
Question
1 Approved Answer
what mass of carbon dioxide is present in 1.00m^3 of dry air at a temperature of 19C and of 685 torr. Calculate the mass percent
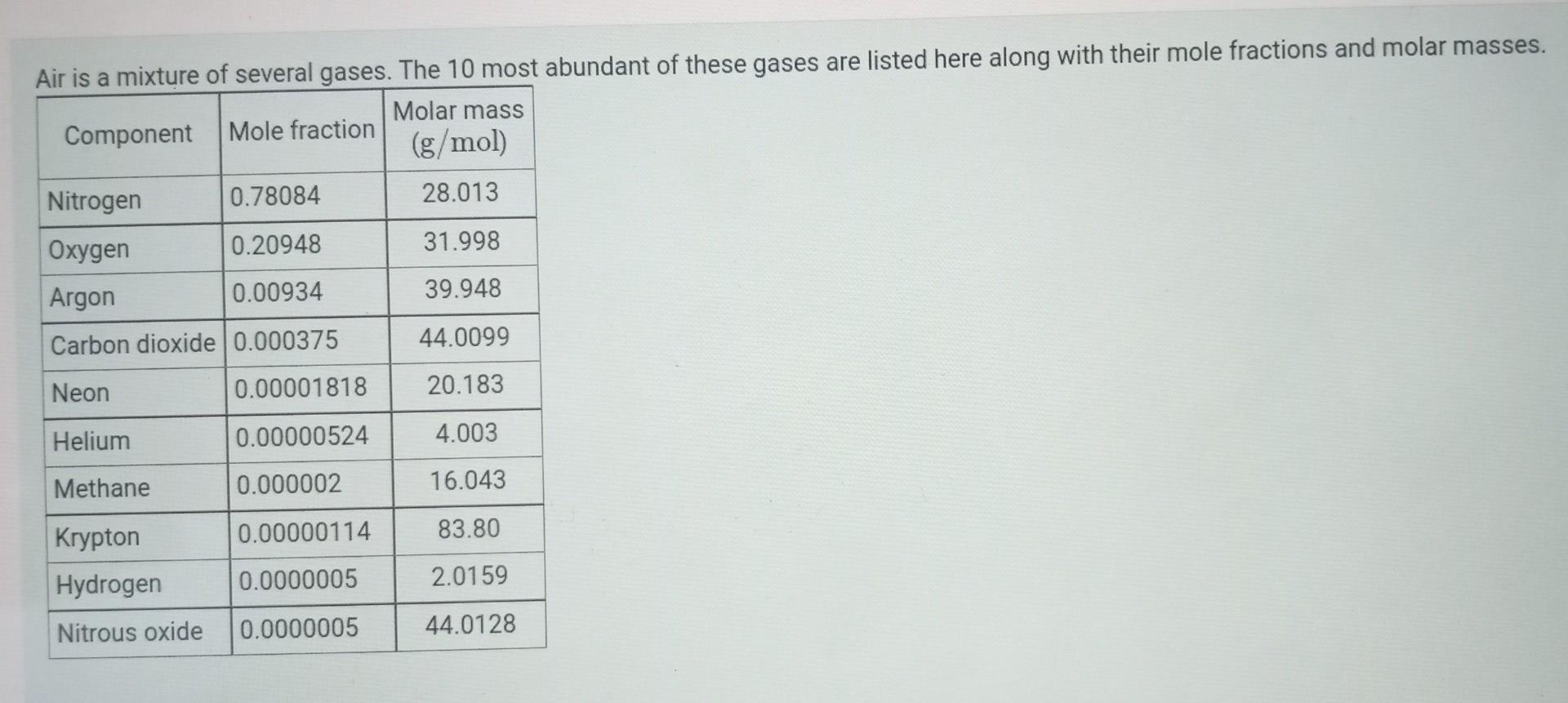
what mass of carbon dioxide is present in 1.00m^3 of dry air at a temperature of 19C and of 685 torr. Calculate the mass percent of oxygen in dry air.
Air is a mixture of several gases. The 10 most abundant of these gases are listed here along with their mole fractions and molar masses. Molar mass Component Mole fraction (g/mol) Nitrogen 0.78084 28.013 Oxygen 0.20948 31.998 Argon 0.00934 39.948 Carbon dioxide 0.000375 44.0099 Neon 0.00001818 20.183 Helium 0.00000524 4.003 Methane 0.000002 16.043 0.00000114 83.80 Krypton Hydrogen 0.0000005 2.0159 Nitrous oxide 0.0000005 44.0128Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


