Question
What volume of a 12.0 M H2SO4 solution would be required to produce 300. mL of 4.0 M H2SO4?
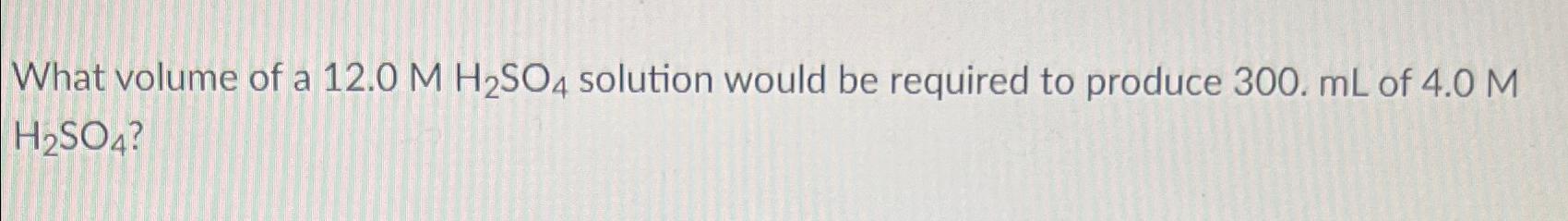
What volume of a 12.0 M H2SO4 solution would be required to produce 300. mL of 4.0 M H2SO4?
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solution Calculating the v...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry Principles And Modern Applications
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
11th Edition
0132931281, 978-0132931281
Students also viewed these Mathematics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



