Answered step by step
Verified Expert Solution
Question
1 Approved Answer
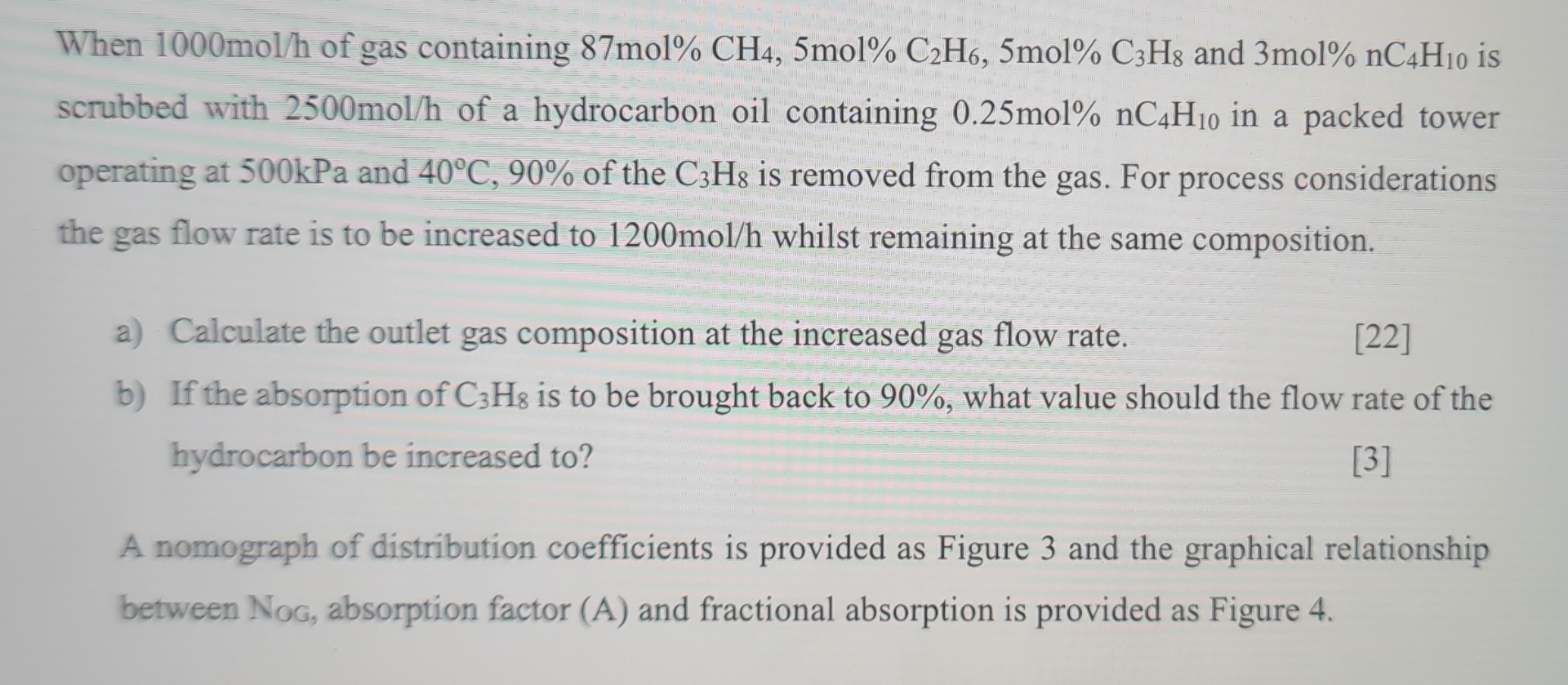
When 1 0 0 0 m o l h of gas containing 8 7 mol % C H 4 , 5 m o l 2
When of gas containing mol and mol is scrubbed with of a hydrocarbon oil containing in a packed tower operating at kPa and of the is removed from the gas. For process considerations the gas flow rate is to be increased to whilst remaining at the same composition.
a Calculate the outlet gas composition at the increased gas flow rate.
b If the absorption of is to be brought back to what value should the flow rate of the hydrocarbon be increased to
A nomograph of distribution coefficients is provided as Figure and the graphical relationship between Nog, absorption factor and fractional absorption is provided as Figure
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


