Answered step by step
Verified Expert Solution
Question
1 Approved Answer
When balancing this equation there are infinite possibilities for b and c values. I got a=2, b=2, and c=2. I am not sure if this
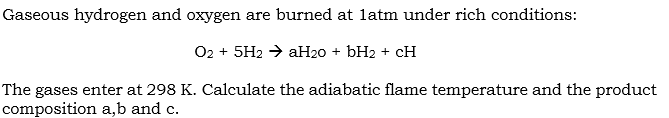
When balancing this equation there are infinite possibilities for b and c values. I got a=2, b=2, and c=2. I am not sure if this is correct and how to proceed from here to find the flame temperature.
please don't post the same answer from the other chegg post
Gaseous hydrogen and oxygen are burned at latm under rich conditions: O2 + 5H2 H20 + bH2 + CH The gases enter at 298 K. Calculate the adiabatic flame temperature and the product composition a,b and cStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


