Question
When ethanol is dehydrogenated in the presence of air over a catalyst, the following chemical reactions occur. The feed to the reactor consists of ethanol
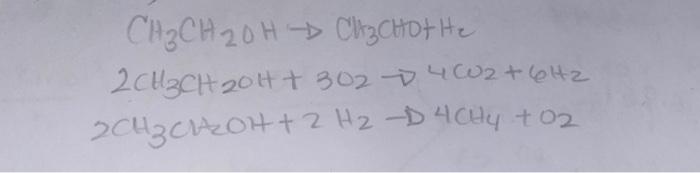
When ethanol is dehydrogenated in the presence of air over a catalyst, the following chemical reactions occur. The feed to the reactor consists of ethanol and air. The product from the reactor goes to a condenser where the acetaldehyde is separated. The rest of the products come out as a mixture of gases whose composition is: 18.81% H2, 8.68% CH4, 54.42% N2, X% CO2 and Y% O2. decide: 1) The number of degrees of freedom of the process is based on 100 moles of gas mixture leaving the reactor. 2) The amount of acetaldehyde produced in moles. 3) The amount of ethanol fed, in moles. 4) The composition (mole %) of CO2 and O2 in the gas mixture leaving the reactor. 5) The percentage conversion of ethanol to acetaldehyde. 6) The selectivity (moles acetaldehyde/moles methane) of acetaldehyde to methane.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


