Question
When the concentration of methane gas (CH4) in the air is between 5-15%, it can cause firestorm explosions. A mine gas flowing at a molar
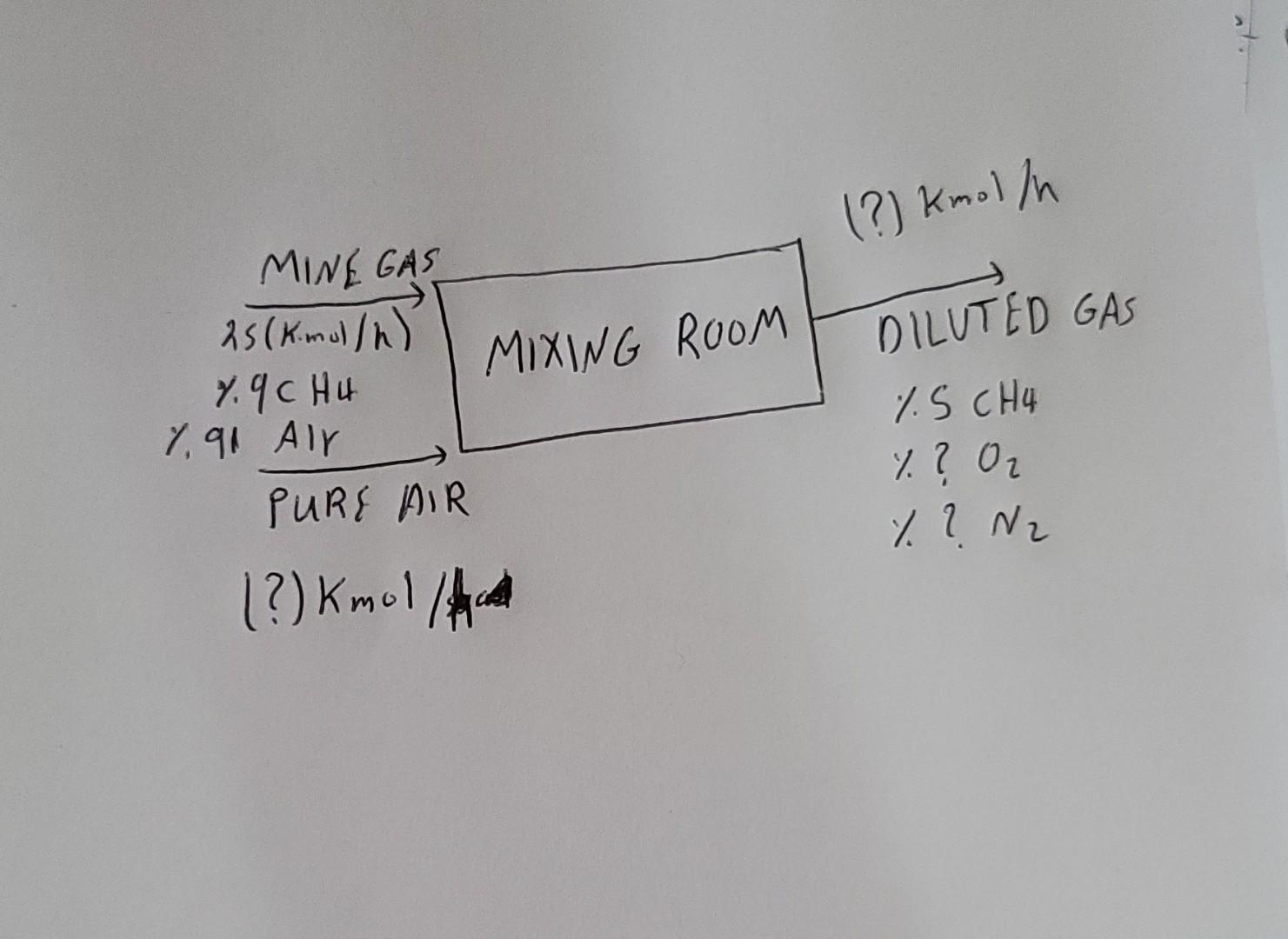
When the concentration of methane gas (CH4) in the air is between 5-15%, it can cause firestorm explosions. A mine gas flowing at a molar flow rate of 25 (kmol/h) and containing 9% CH4+91% dry air by volume is diluted by mixing with the pure air stream to eliminate the risk of explosion. Since steady state conditions are valid; a) Calculate the minimum pure air molar flow rate (kmol/hour) required for dilution. b) Calculate the molar flow rate and the vol% O2 and N2 concentrations for the case where the diluted stream leaving the mixing chamber contains 5% methane.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


