Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Which molecule has given rise to the spectra shown below. Justify your answer with respect to IR, 1 H NMR and 13 C NMR. Keep
Which molecule has given rise to the spectra shown below. Justify your answer with respect to IR, 1 H NMR and 13 C NMR. Keep in mind that Hs that switch with D2O usually do not show connections to other nearby H. The integrals in HNMR are marked in red text. Below the image with spectral data, you will find various shift tables for NMR IR etc. 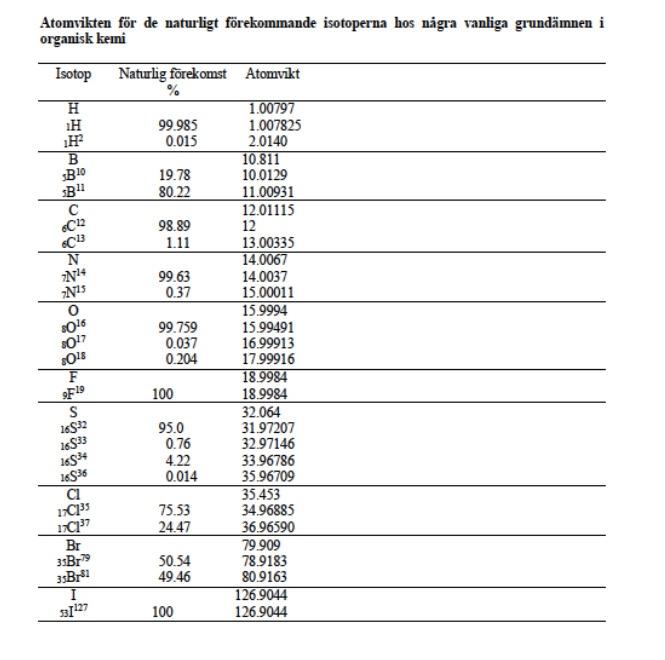
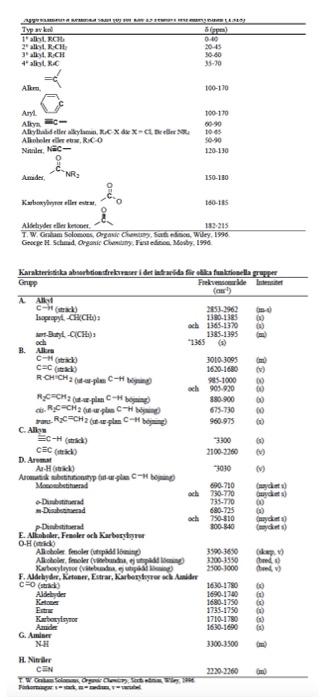
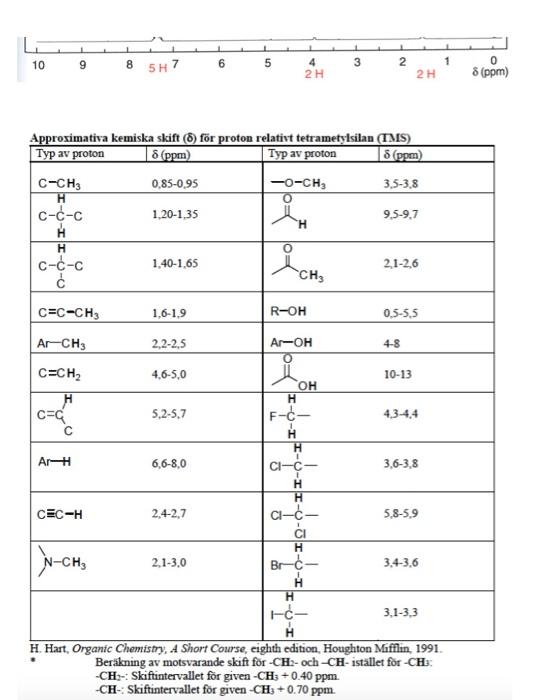
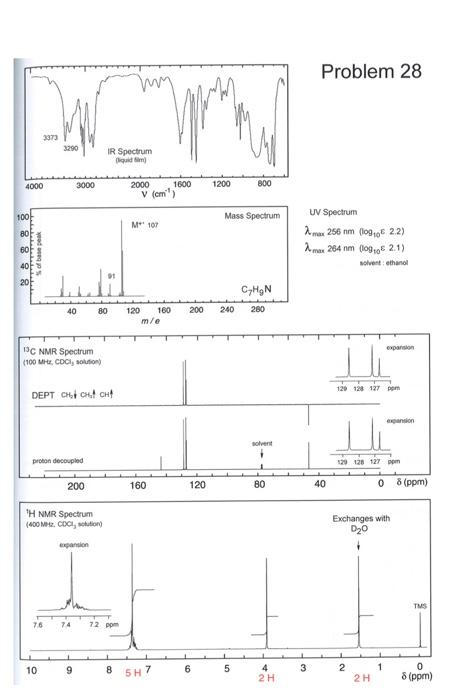
B 10 B! 6C12 6C13 N NH 2015 Atomvikten fr de naturligt frekommande isotoperna hos ngra vanliga grundmnen i organisk kemi Isotop Naturlig forekomst Atomvikt % 1.00797 1H 99.985 1.007825 0.015 2.0140 10.811 19.78 10.0129 80.22 11.00931 12.01115 98.89 12 1.11 13.00335 14.0067 99.63 14.0037 0.37 15.00011 15.9994 99.759 15.99491 0.037 16.99913 0.204 17.99916 18.9984 100 18.9984 32.064 95.0 31.97207 0.76 32.97146 4.22 33.96786 0.014 35.96709 35.453 75.53 34.96885 24.47 36.96590 Br 79.909 3sBr 50.54 78.9183 49.46 80.9163 126.9044 100 126.9044 O 2016 2017 3018 F F19 S 16532 16533 16534 16936 1113s 17" 35Brs I 33127 AY Typ av 1.CH 2. RCH 3. R.CH 4 ayt. Spes) 040 30 60 35-30 Akr. 100-170 Aryl Alky Alkydeber.CXX Alieledw.co Nac- 100-170 1065 120-130 Amader NR 150-180 Karbonile este 160-165 Alderyderelles con 182 215 T. W. Gribam Solomons, Organe Chancen for den Wiley, 1990 George HL Schmid. Organic Chemistry Fest edited Mosby, 1996 iss1 18 33 338 38 cec Karakteristianismes i det tid for a funktionella papper Gryp Frekvenser de neste (a) AA C- 2853-1962 Isopropyl CHLO 1330-1385 och 1365-1370 Buy-OCHE) 1385-1395 och *2.365 B. Allure C- 3010 3095 CEC 1620-1680 ) RCHICH2C- 985-1000 och 903 920 RC CH2-plan-ing 880-900 RC CH2CH 675-730 RC-CH 2 place 960-975 C. Als Ec- 3300 2100-2350 D. Ar Ar-H) 9030 Armut betale Mobetianad 690-710 och 790-770 Gay +Dham | 735-70 00 -Dhammad 680-725 (1) och 790-310 my pDubad 300-840 (d) E. AlkoholerFealer och arbetsu O-Hnid Aloolfole (tipid Long 35903850 ke Ako melere, pa 3200-3550 theo Xarboxyleyser (vibe 2500-1000 F. Aldehyder, Ketone, Estrar, Karbergerac Ander C G 1680-1780 Aides 1690-17:00 1680-1750 Extra 1735-1750 Karbasyim 1710-1780) 1630-1600 NH 3300-3500 H. Nie CEN 2220 2360 om TV Fadi. 888888 Amide G Am 1 1 10 9 6 8 5H7 5 3 1 2 4 2 H 0 8 (ppm) 2H 8 (ppm) Approximativa kemiska skift() fr proton relativt tetrametylsilan (IMS) Typ av proton Typ av proton 8 (ppm) C-CH3 0.85-0,95 -O-CH3 3,5-3,8 H O C-C-c 1.20-1,35 9.5-9,7 H H C--c 1.40-1,65 2.1-2,6 . O C=C-CH3 1.6-1.9 R-OH 0.5-5.5 Ar-CH3 2,2-2,5 ArOH 4-8 4,6-5,0 10-13 C=CH2 H CEC 5,2-5,7 4.3-4.4 Ar- 6,6-8,0 OH H F-- H H CI-C- 1 H H C-- CI H 3,6-3,8 TE CEC-H 2,4-2,7 5,8-5,9 N-CH - 2.1-3.0 Br- 3.4-3,6 H H EC 3.1-3,3 H. Hart, Organic Chemistry, A Short Course, eighth edition, Houghton Mifflin. 1991. Berkning av motsvarande skift for-CH2-och-CH- istllet for -CH: -CH-Skiftintervallet fr given -CH3 +0.40 ppm. -CH-Skiftintervallet fr given-CH3 + 0.70 ppm. Problem 28 w 3373 3290 IR Spectrum ladim M 4000 3000 1200 800 2000 1800 V (cm) 100! Mass Spectrum M107 80F 8 8 8 8 of base peak UV Spectrum 2 mar 256 nm (10906 2.2) max 264 nm (109,06 2.1) solvent: ethanol Luli CHON 280 40 80 200 240 120 180 mle expansion 13C NMR Spectrum (100 M COCI, 129 128 127 pm DEPT CHICH non solvent 1 proton decoupled 129 128 127 1 200 160 120 80 40 0 8 8 (ppm) TH NMR Spectrum (400 M2. CDC, stond Exchanges with D20 expansion TMS 76 74 72 ppm 1 10 9 8 6 5 3 1 5H7 1 2 2 H 4 2H 0 8 (ppm) 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


