Question
Which of the following is not an oxidation-reduction reaction? a) 2 Na(s) + Br(g) 2 NaBr(g) b) 2 C(s) + O(g) 2 CO(g) c)
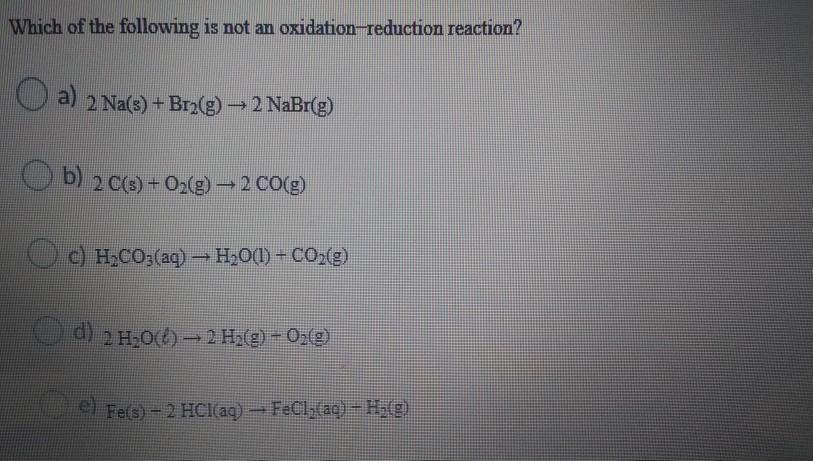
Which of the following is not an oxidation-reduction reaction? a) 2 Na(s) + Br(g) 2 NaBr(g) b) 2 C(s) + O(g) 2 CO(g) c) HCO3(aq) HO(l) + CO(g) 2 HO()-2 H(g) - O(g) el Fe(s)-2 HCl(aq) FeCl(aq) - H(g)
Step by Step Solution
3.39 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
The detailed answer for the above question is provide...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Accounting concepts and applications
Authors: Albrecht Stice, Stice Swain
11th Edition
978-0538750196, 538745487, 538750197, 978-0538745482
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



