Question
Question 4 Consider the following reaction: CH(g) + 2H(g) -> CH6(g) If the instantaneous initial rate of reaction is 1.45 x 10-4 M/s, calculate
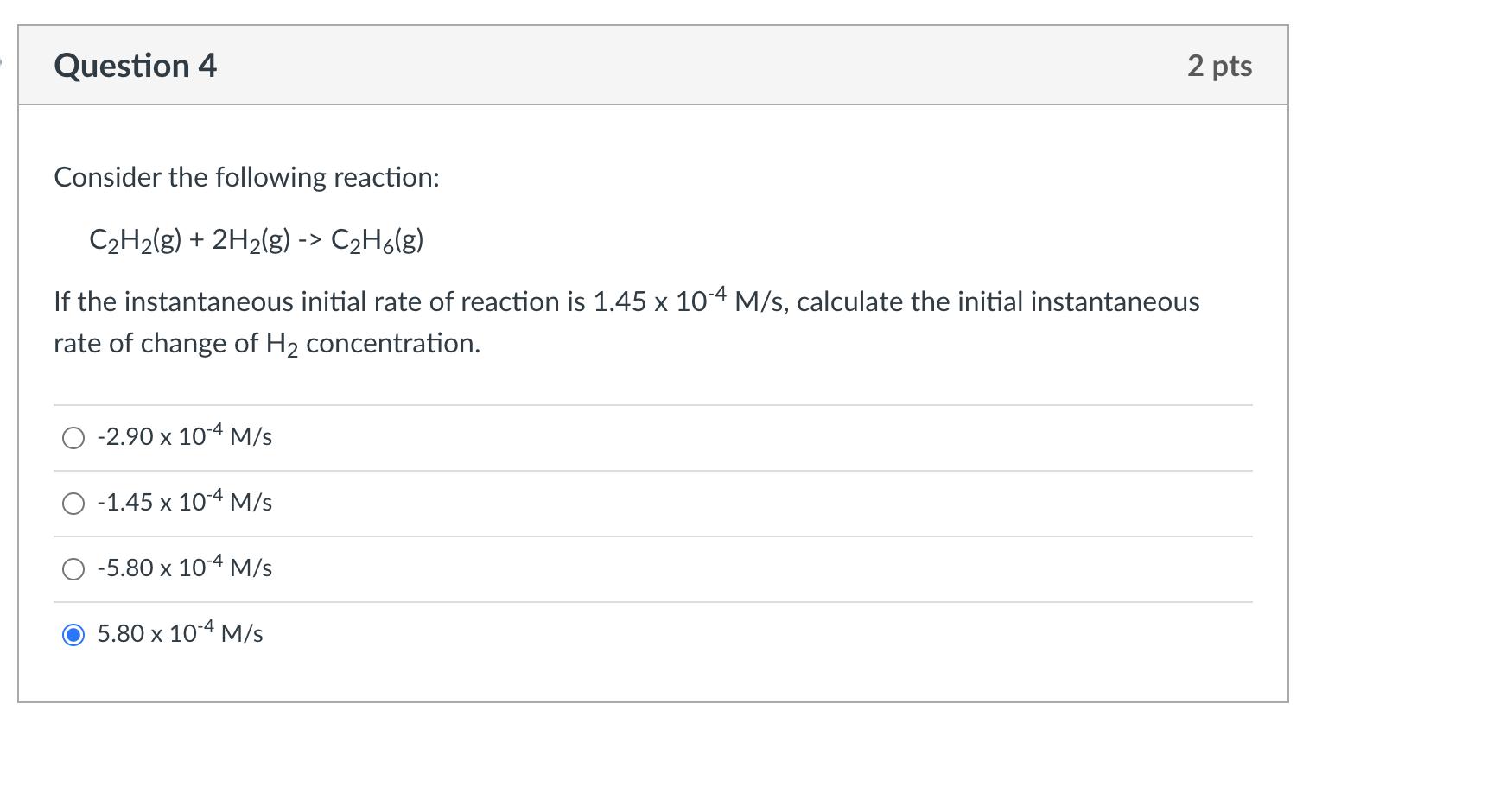
Question 4 Consider the following reaction: CH(g) + 2H(g) -> CH6(g) If the instantaneous initial rate of reaction is 1.45 x 10-4 M/s, calculate the initial instantaneous rate of change of H concentration. -2.90 x 10-4 M/s -1.45 x 10-4 M/s -5.80 x 10-4 M/s 2 pts 5.80 x 10-4 M/s
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
Correct option is A As the reaction proceed the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managers and the Legal Environment Strategies for the 21st Century
Authors: Constance E. Bagley
7th edition
1111530637, 978-1285319636, 978-1111530631
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



