Answered step by step
Verified Expert Solution
Question
1 Approved Answer
While in principle we could attempt to solve for the exact equations that correspond to the energy levels of different substances, this is extremely complicated.
While in principle we could attempt to solve for the exact equations that correspond to the energy levels of
different substances, this is extremely complicated. However, it is often simpler to determine these energy
levels experimentally. We can excite a gas electrically so that the electrons in the various orbitals will jump
up to a higher energy state, and thus regularly decay to lower energy states, thereby emitting photons as
above. If the energy levels are quantized, then we will see a discrete set of colors, which generally combine
to form a single color. We can then use a diffraction grating to separate these colors; they diffract at different
angles based on their wavelength according to Eq and it is then simple to measure these angles.
We can test our understanding of this by using the grating spectrometer to study the spectral lines of hydro
gen, to reproduce the known both theoretically and empirically spectrum. Additionally, we can calibrate
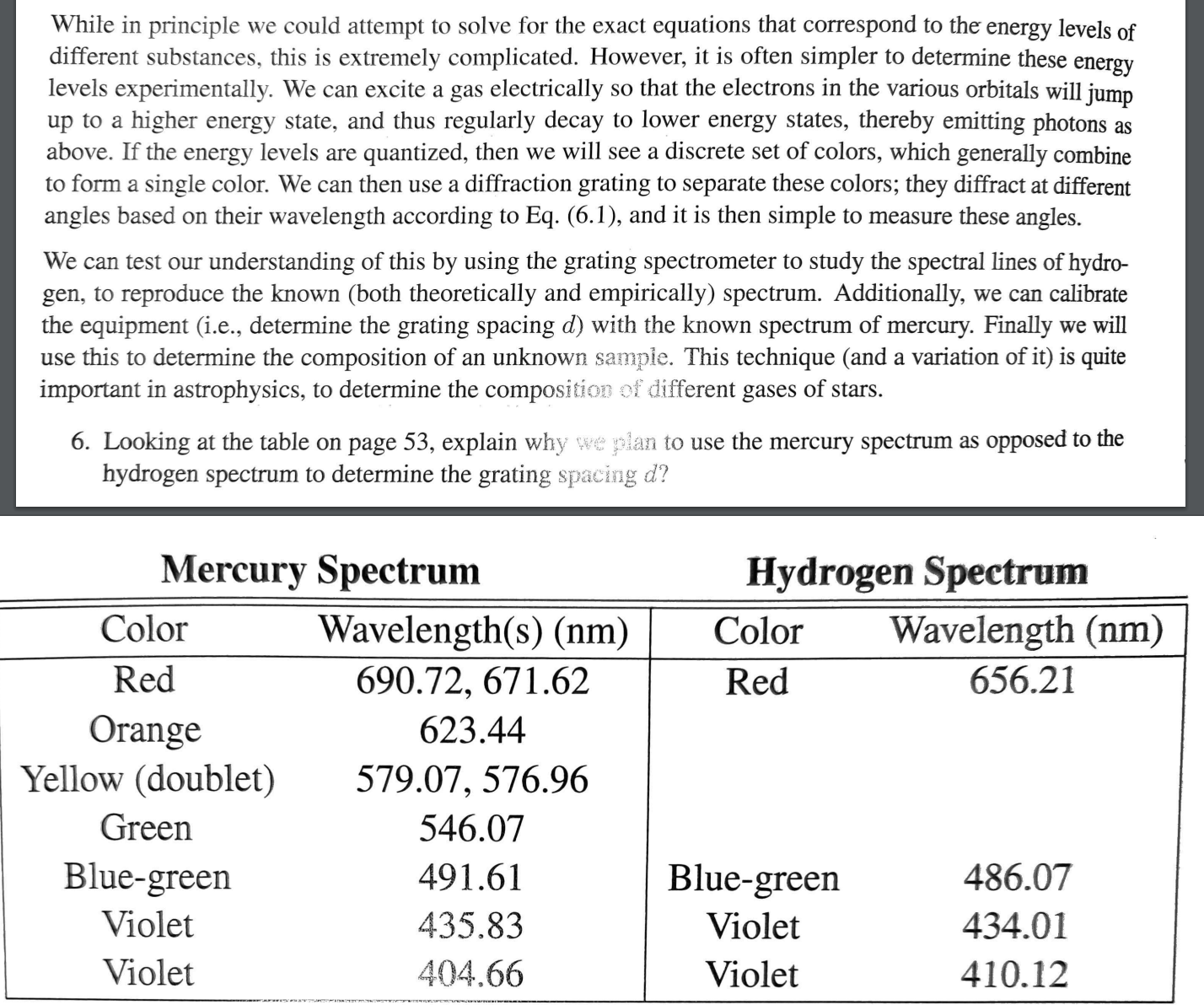
the equipment ie determine the grating spacing with the known spectrum of mercury. Finally we will
use this to determine the composition of an unknown sample. This technique and a variation of it is quite
important in astrophysics, to determine the composition of different gases of stars.
Looking at the table on page explain why when to use the mercury spectrum as opposed to the
hydrogen spectrum to determine the grating spacing
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


