Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Why is the ion you chose in the above question the least stable? a) negative charge is on the most electronegative atom b) negative charge
Why is the ion you chose in the above question the least stable?
a) negative charge is on the most electronegative atom
b) negative charge is on the least electronegative atom
c) negative charge is on the largest atom
d) negative charge is on the smallest atom
e) effective charge is reduced the most by an inductive effect
f) effective charge is reduced the least by an inductive effect
g) charge is delocalized by resonance
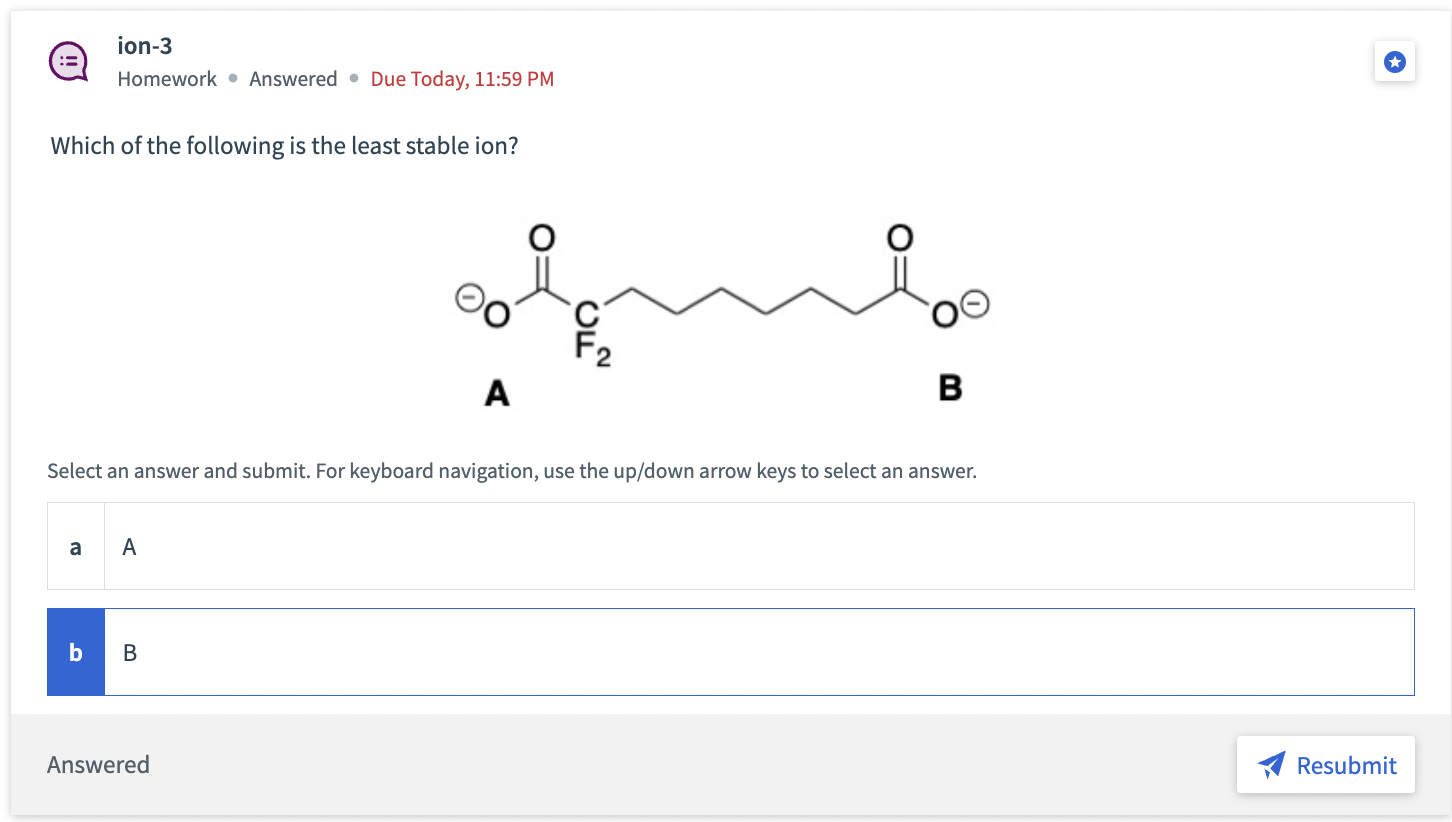
ion-3 Homework Answered - Due Today, 11:59 PM Which of the following is the least stable ion? Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select anStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


