WILL UPVOTE

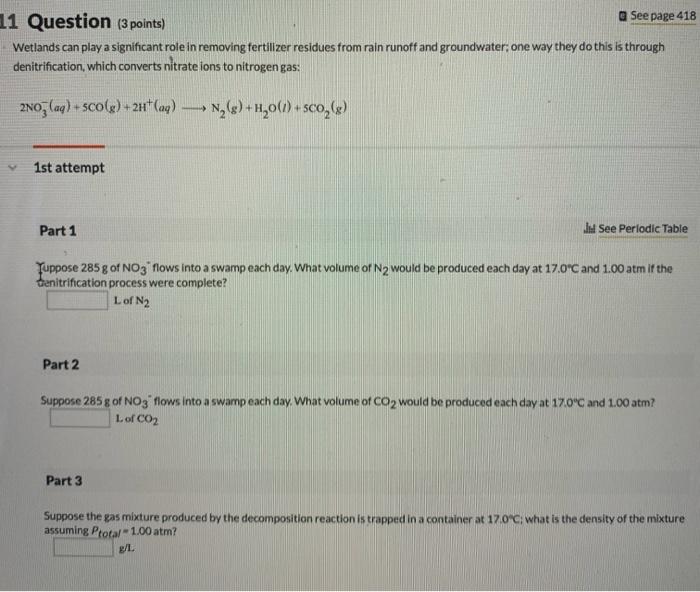
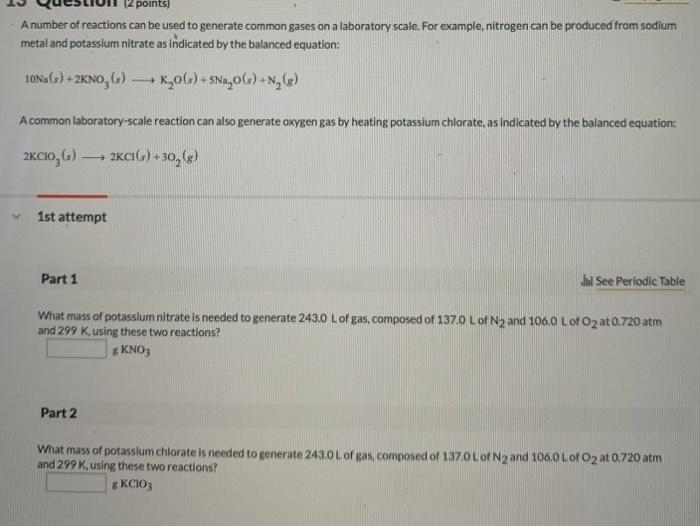
A flask of ammonia is connected to a flask of an unknown acid HX by a 1.46m glass tube (where "X" represents a halogen). As the two gases diffuse down the tube, a white ring of NH4X forms 100cm from the ammonia flask. Identify element X (Name or symbol). If 0.160 mol of an ideal gas has a volume of 1929mL and a pressure of 5.50atm, what is its temperature in degrees Celsius? Use one of the following values: R=0.0821atmL/molKR=8.31kPaL/molKR=62.4torrL/molK A gas composed of chlorine and oxygen has a density of 2.875g/L at 759.5mmHg and 11C. What is the most likely molecular formula of the gas? Wetlands can play a significant role in removing fertilizer residues from rain runoff and groundwater; one way they do this is through denitrification, which converts nitrate ions to nitrogen gas: 2NO3(aq)+5CO(g)+2H+(aq)N2(g)+H2O(l)+5CO2(g) 1st attempt Part 1 H See Periodic Table Yuppose 285g of NO3 flows into a swamp each day. What volume of N2 would be produced each day at 17.0C and 1.00 atm if the tenitrification process were complete? L of N2 Part 2 Supoose 285 a of NO3; flows into a swamp each day. What volume of CO2 would be produced each day at 17.0C and 1.00 atm? L of CO2 Part 3 Suppose the gas mixture produced by the decomposition reaction is trapped in a container at 170C; what is the density of the mixture assuming Ptafal = 1.00atm ? g/L A number of reactions can be used to generate common gases on a laboratory scale. For example, nitrogen can be produced from sodium metal and potassium nitrate as indicated by the balanced equation: 10Na(s)+2KNO3(s)K2O(s)+5Na2O(s)+N2(g) A common laboratory-scale reaction can also generate oxygen gas by heating potassium chiorate, as indicated by the balanced equation: 2KClO3(s)2KCI(s)+3O2(g) 1st attempt Part 1 Wh See Periodic Table What mass of potassium nitrate is needed to generate 243.0L of gas, composed of 137.0L of N2 and 106.0L of O2 at 0.720 atm and 299 K. using these two reactions? g. KNO3 Part 2 What mass of potassium chiorate is needed to generate 243.0L of gas, composed of 137.0L of N2 and 106.0L1 of O2 at 0.720 atm and 299K, using these two reactions? g KClO3