Question
With respect to the compounds I-V, choose the correct statement(s). H H H-CH3 H H=H || IV V (A) The acidity of compound I
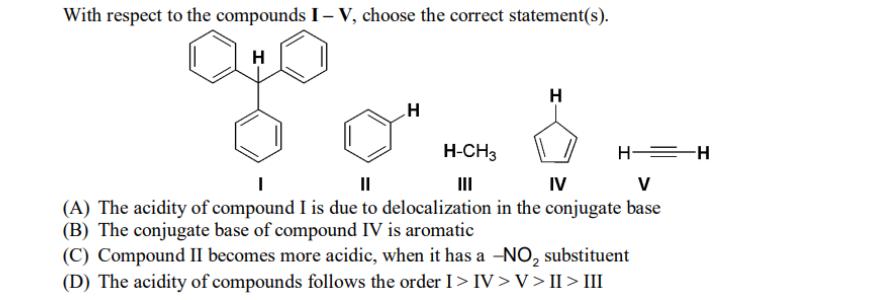
With respect to the compounds I-V, choose the correct statement(s). H H H-CH3 H H=H || IV V (A) The acidity of compound I is due to delocalization in the conjugate base (B) The conjugate base of compound IV is aromatic (C) Compound II becomes more acidic, when it has a -NO substituent (D) The acidity of compounds follows the order I > IV>V>II>III
Step by Step Solution
3.32 Rating (140 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: John McMurry
7 Edition
978-0495112587, 0495112585
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



