Answered step by step
Verified Expert Solution
Question
1 Approved Answer
with steps for each please Table 1: Standardization Data Trial 1 Trial 2 Trial Mass of KHP 0.5890 0.5825 0.5818 Initial burette reading 0.20ml 16.90mL
with steps for each please 
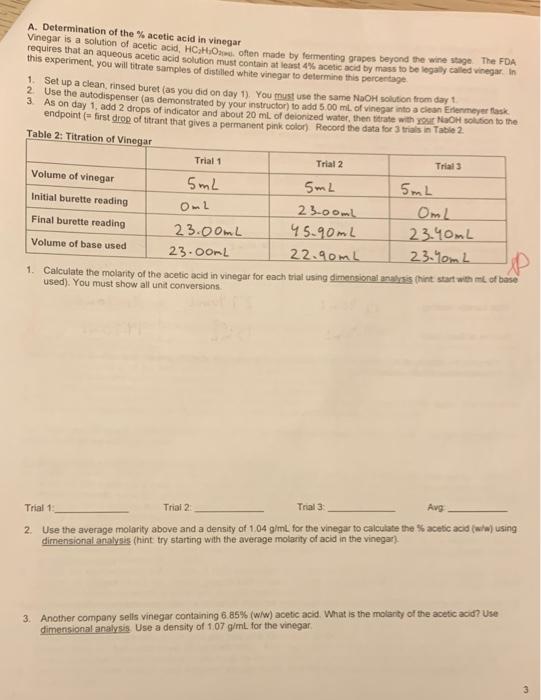
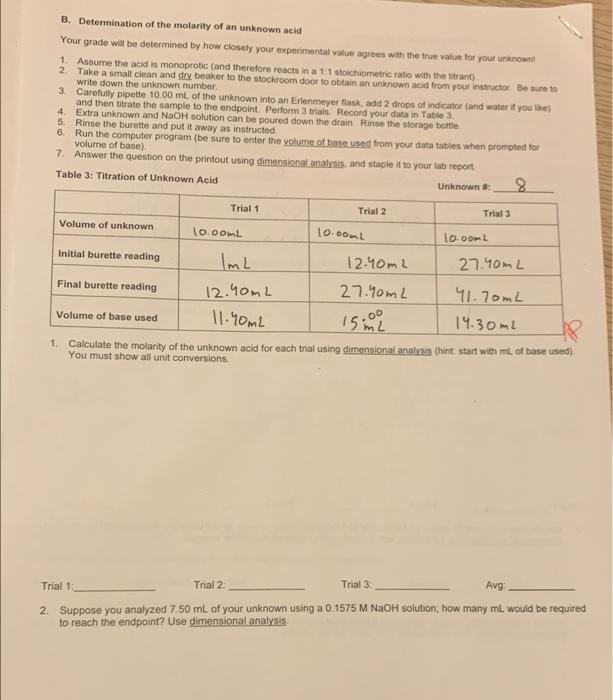
Table 1: Standardization Data Trial 1 Trial 2 Trial Mass of KHP 0.5890 0.5825 0.5818 Initial burette reading 0.20ml 16.90mL 31.90mL Final burette reading 16.90ml 31.90mL 96.90mL Volume of base used 16.70mL 15.00mL 15.00mL Data Analysis: 1. Write a balanced molecular equation (with phases, of course) for the reaction between the KHP and the trant NaOH(aq) + KHC & Hy Oy KN a C a Hyo HDD - yonid 2 Write the net ionic equation for this reaction (note that HCH.O is a weak acid) Be sure to include phases HC49 HChan Olliams HO (2) + Cg Hyou (4) 0.5825g * 201.2333 Ime 3. Calculate the molarity of base for each trial. You must use dimensional analysis no other methods or short cuts wil be accepted, so show all unit conversions (hint: start with grams of KHP used) 10.5890 g 204.223, 204.223 -2 0.58187 20.00 288 mol = 0.00285-1 -0.00284 15.00mL : 0.015L 15. OnL20.0SL 16.70m2 = 0.017L 1L = 1000mL IL ODL L=1000mL M: 0.6022" 0.89M 0.015 M: 0.00295 0.017-0.169M M2000795 = 0.19 Trial _0.169 M Trial 2 0.19 M Trial 30-189 M If one of the trials differs significantly from the others, consult your instructor 4. Calculate the average molarity of the base: Average: 0.1$2M USE THIS AVERAGE MOLARITY OF THE BASE FOR THE DATA ANALYSIS ON PAGES 3 & 4 5. Using dimensional analysis, calculate the volume of your NaOH solution needed to titrate 2.45 g of KHP A. Determination of the % acetic acid in vinegar Vinegar is a solution of acetic acid, HCM, often made by fermenting grapes beyond the wine star The FDA requires that an aqueous acetic acid solution must contain at least 4% acetic acid by mass to be legaly called vinegar. In this experiment, you will fitrate samples of distilled white vinegar to determine this percentage 1 Set up a clean, rinsed buret (as you did on day 1). You must use the same NaOH solution from day ? Use the autodispenser (as demonstrated by your instructor to add 5,00 ml. of vinegar into a clean Ertemeyer flask 3 As on day 1, add 2 drops of indicator and about 20 mL of deionized water, then titrate with you NaOH in to the endpoint (= first drop of titrant that gives a permanent pink colon Record the data for 3 trials in Table 2 Table 2: Titration of Vinegar Trial 1 Trial 2 Trial Volume of vinegar 5mL 5mL 5mL Initial burette reading Om2 23.00 OmL Final burette reading 23.00mL 45.90mL 23.40mL Volume of base used 23.OOL 22.90mL 23.0mL 1. Calculate the molarity of the acetic acid in vinegar for each trial using dimensional ass hint start with me of base used). You must show all unit conversions D Trial 1 Trial 2 Trial 3 Avg 2. Use the average molarity above and a density of 104 g/ml. for the vinegar to calculate the acetic acid (w/w) using dimensional analysis (hint try starting with the average molarity of acid in the vinegar) 3. Another company sells vinegar containing 6 85% (w/w) acetic acid. What is the molarity of the acetic acid? Use dimensional analysis. Use a density of 107 g/mL. for the vinegar B. Determination of the molarity of an unknown acid Your grade will be determined by how closely your experimental value agrees with the true value for your known 1. Assume the acid is monoprotic (and therefore reacts in a 1:1 stoichiometric ratio with the titrant) 2. Take a small clean and dry beaker to the stockroom door to obtain an unknown acid from your instructor Be sure to write down the unknown number 3. Carefully pipette 10.00 mL of the unknown into an Erlenmeyer flask, add 2 drops of indicator and water if you like and then titrate the sample to the endpoint. Perform 3 trial Record your data in Table 3 4 Extra unknown and NaOH solution can be poured down the drain. Rinse the storage bottle 5. Rinse the burette and put it away as instructed 6. Run the computer program (be sure to enter the volume of base used from your datatables when prompted for volume of base) 7. Answer the question on the printout using dimensional analysis, and staple it to your tab report Table 3: Titration of Unknown Acid Unknown Trial 1 Trial 2 Trials 10.com Volume of unknown 10.00mL 10.comL Initial burette reading ImL 12.40mL 27.40mL Final burette reading 12.40mL 27.40mL 41.70mL Volume of base used 11.90ml isina 14.30m2 1. Calculate the molarity of the unknown acid for each trial using dimensional analysis (hint start with mL of base used) You must show all unit conversions Trial 1 Trial 2 Trial 3: Avg 2. Suppose you analyzed 7.50 mL of your unknown using a 0.1575 M NaOH solution, how many mL would be required to reach the endpoint? Use dimensional analysis 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


