Answered step by step
Verified Expert Solution
Question
1 Approved Answer
With the above data and information, plot the calibration curve, and also Calculate conversions using the value of conductivity. Experiment: Continuous Stirred Tank Reactor (CSTR)
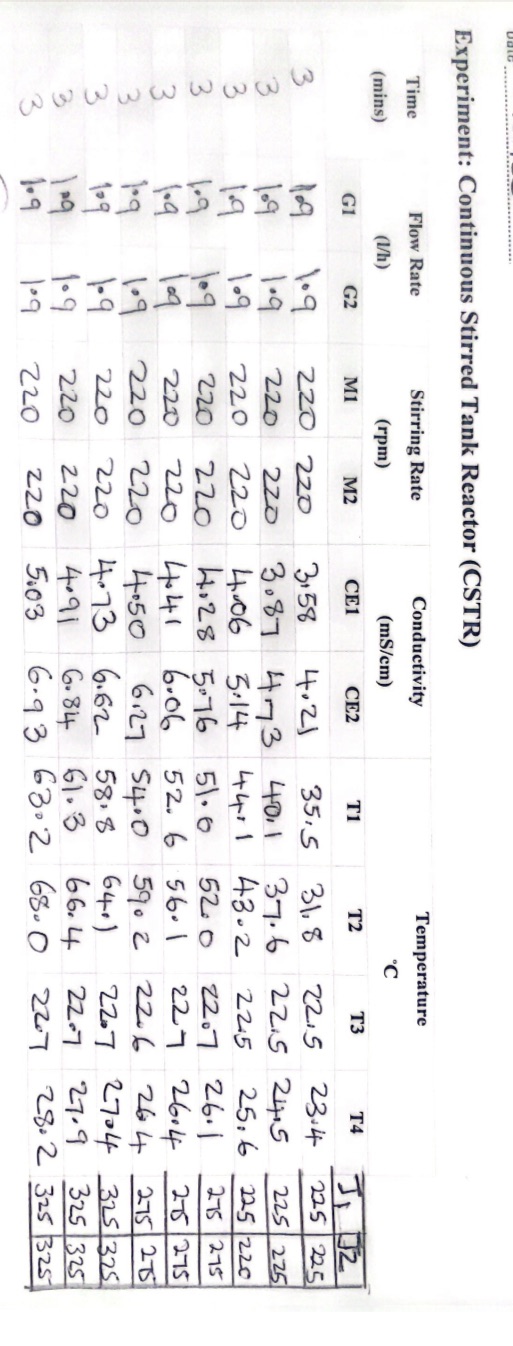
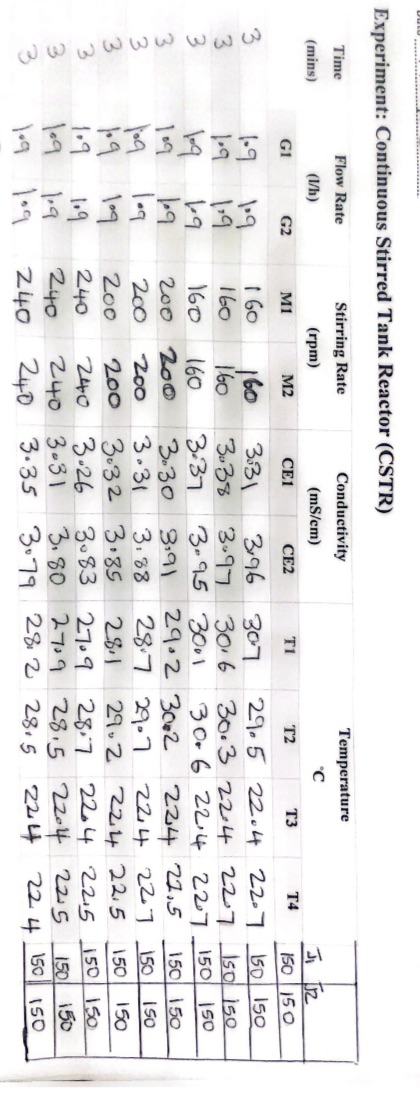
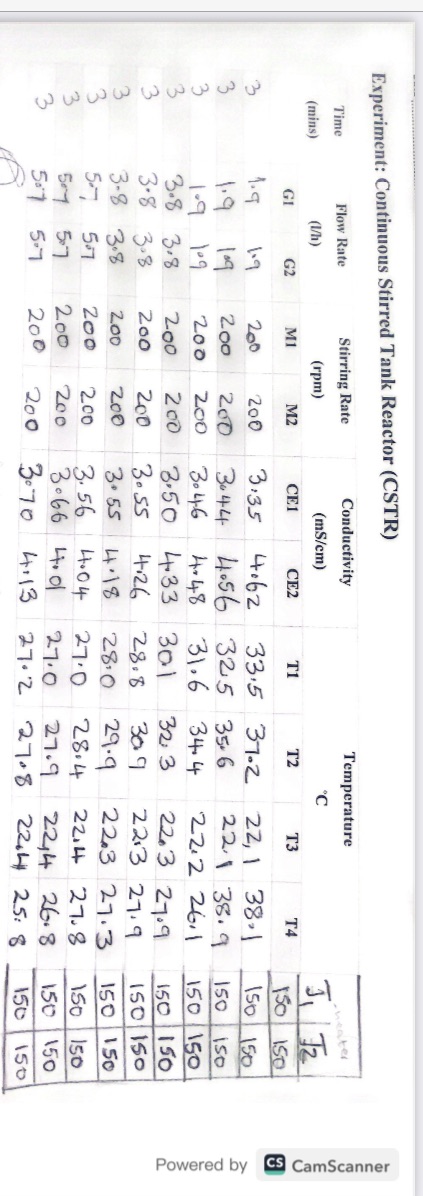
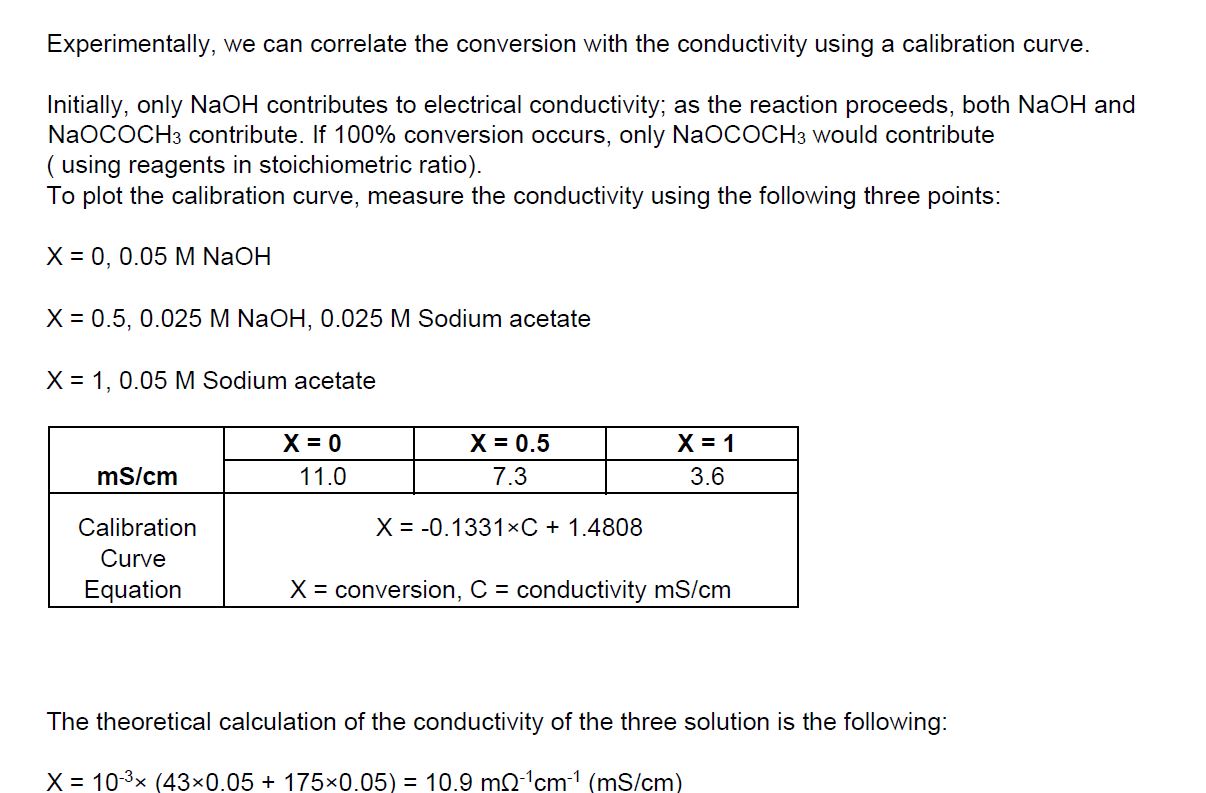
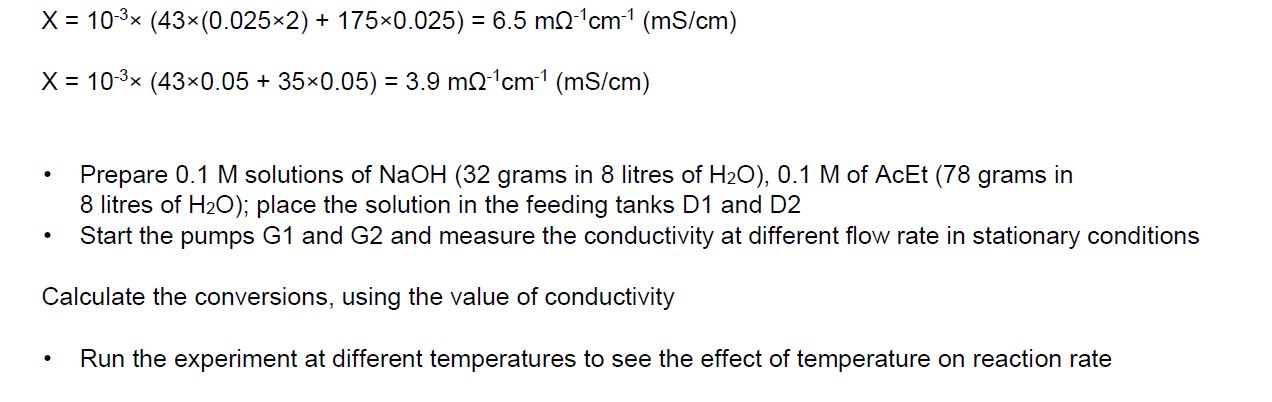
With the above data and information, plot the calibration curve, and also Calculate conversions using the value of conductivity.
Experiment: Continuous Stirred Tank Reactor (CSTR) Experiment: Continuous Stirred Tank Reactor (CSTR) Experiment: Continuous Stirred Tank Reactor (CSTR) Experimentally, we can correlate the conversion with the conductivity using a calibration curve. Initially, only NaOH contributes to electrical conductivity; as the reaction proceeds, both NaOH and NaOCOCH3 contribute. If 100% conversion occurs, only NaOCOCH3 would contribute ( using reagents in stoichiometric ratio). To plot the calibration curve, measure the conductivity using the following three points: X=0,0.05MNaOHX=0.5,0.025MNaOH,0.025MSodiumacetateX=1,0.05MSodiumacetate The theoretical calculation of the conductivity of the three solution is the following: X=103(430.05+1750.05)=10.9m1cm1(mS/cm) X=103(43(0.0252)+1750.025)=6.5m1cm1(mS/cm)X=103(430.05+350.05)=3.9m1cm1(mS/cm) - Prepare 0.1M solutions of NaOH (32 grams in 8 litres of H2O ), 0.1M of AcEt ( 78 grams in 8 litres of H2O ); place the solution in the feeding tanks D1 and D2 - Start the pumps G1 and G2 and measure the conductivity at different flow rate in stationary conditions Calculate the conversions, using the value of conductivity - Run the experiment at different temperatures to see the effect of temperature on reaction rate Experiment: Continuous Stirred Tank Reactor (CSTR) Experiment: Continuous Stirred Tank Reactor (CSTR) Experiment: Continuous Stirred Tank Reactor (CSTR) Experimentally, we can correlate the conversion with the conductivity using a calibration curve. Initially, only NaOH contributes to electrical conductivity; as the reaction proceeds, both NaOH and NaOCOCH3 contribute. If 100% conversion occurs, only NaOCOCH3 would contribute ( using reagents in stoichiometric ratio). To plot the calibration curve, measure the conductivity using the following three points: X=0,0.05MNaOHX=0.5,0.025MNaOH,0.025MSodiumacetateX=1,0.05MSodiumacetate The theoretical calculation of the conductivity of the three solution is the following: X=103(430.05+1750.05)=10.9m1cm1(mS/cm) X=103(43(0.0252)+1750.025)=6.5m1cm1(mS/cm)X=103(430.05+350.05)=3.9m1cm1(mS/cm) - Prepare 0.1M solutions of NaOH (32 grams in 8 litres of H2O ), 0.1M of AcEt ( 78 grams in 8 litres of H2O ); place the solution in the feeding tanks D1 and D2 - Start the pumps G1 and G2 and measure the conductivity at different flow rate in stationary conditions Calculate the conversions, using the value of conductivity - Run the experiment at different temperatures to see the effect of temperature on reaction rateStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


