Question
With the given data, answer the following questions:1. Did the metal or the water have the greater change in temperature? (Answer this for trial 1,
With the given data, answer the following questions:1. Did the metal or the water have the greater change in temperature? (Answer this for trial 1, 2, & 3)2. In step 3 of the calculations, we used the same number for "heat lost by the metal" and "heat gained by the water." Explain why these two numbers are the same.3. Explain why the temperature change of the metal is much larger than the temperature change of the water if the quantity of heat lost by the metal is the same as the quantity of heat gained by the water. Use one of the trials as a specific example. Use your data to help justify your conclusion. ?4. Compare the specific heat of water to the specific heat of the metal. Which is bigger? (Answer this question for all 3 separate trials) 5. What does a higher specific heat indicate about a substance in terms of energy gained and temperature change?
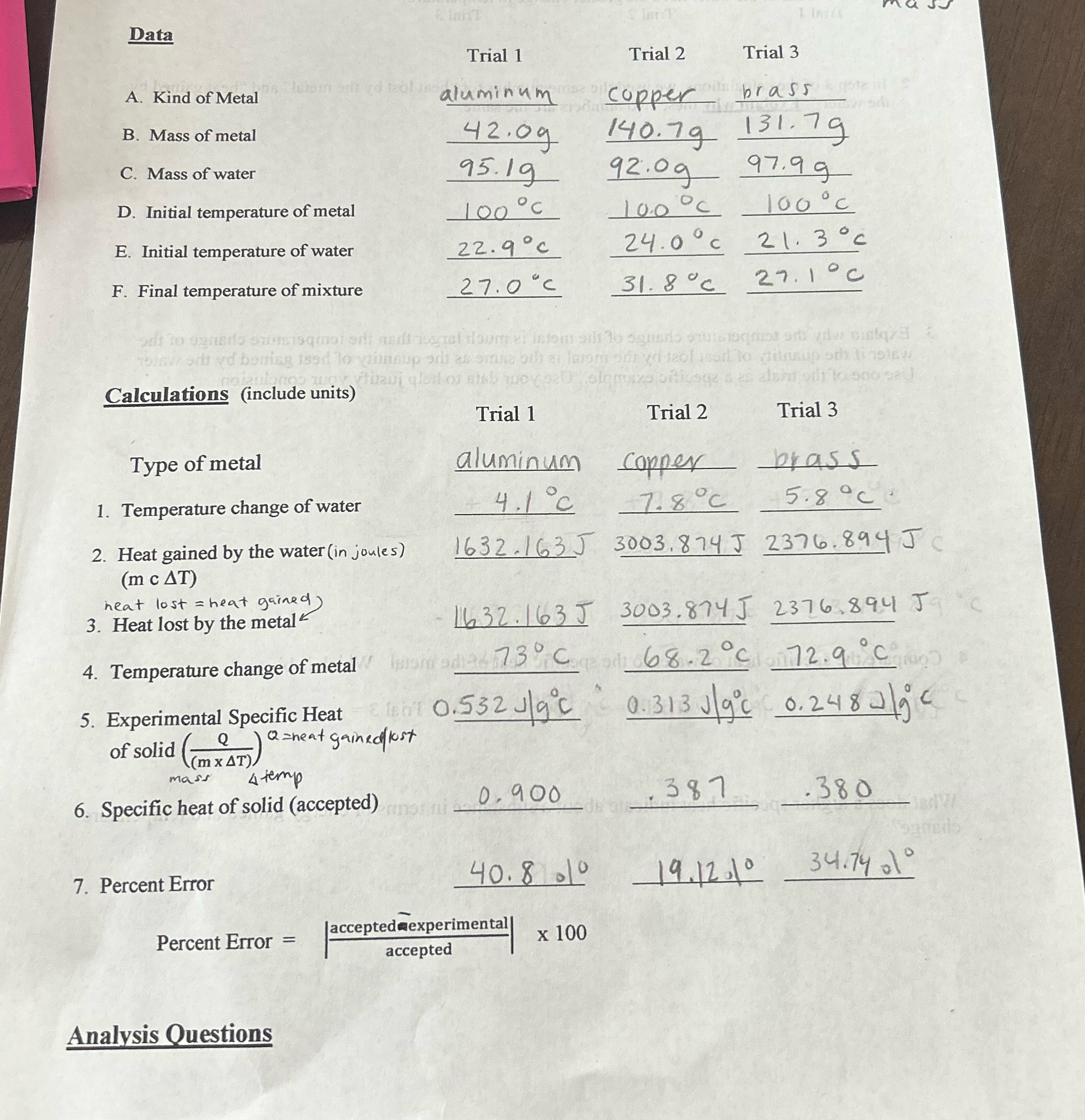
Data lenT Istom styd 120! A. Kind of Metal Trial 1 aluminum Trial 2 Trial 3 brass B. Mass of metal C. Mass of water D. Initial temperature of metal copper 42.09 140.79 131.79 92.09 97.99 95.19 100C 100 C E. Initial temperature of water F. Final temperature of mixture 22.9c 27.0C 100C 24.0C 21.3c 31.8C 27.1C od to ogni siqmated; andi ogrel doum e info at To ognado suisoqmat srt dista To odd boning ised to insup di asemsa oth ei laiem odd seol is to insup oth inslow Calculations (include units) alshi Type of metal Trial 1 Trial 2 aluminum Copper 4.1 C 7.8C Trial 3 brass 5.8C 1. Temperature change of water 2. Heat gained by the water (in joules) (m c AT) heat lost = heat gained) 3. Heat lost by the metal 4. Temperature change of metal 5. Experimental Specific Heat Q of solid (AT (mx AT), mass 1632.163J 3003.874 J 2376.894 JC HOT 0.552 J/gc 8 feb Q=heat gained lost 4 temp 6. Specific heat of solid (accepted) 1632.163J 3003.874 J 2376.894 J Isionad 73C 68.2C 72.9C 0.313J/9c 0.2482/ijc 387 .380 Jad 34.7401 0.900 40.8 1 19.12.10 7. Percent Error Percent Error Jaccepted experimental == x 100 accepted Analysis Questions
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


