Answered step by step
Verified Expert Solution
Question
1 Approved Answer
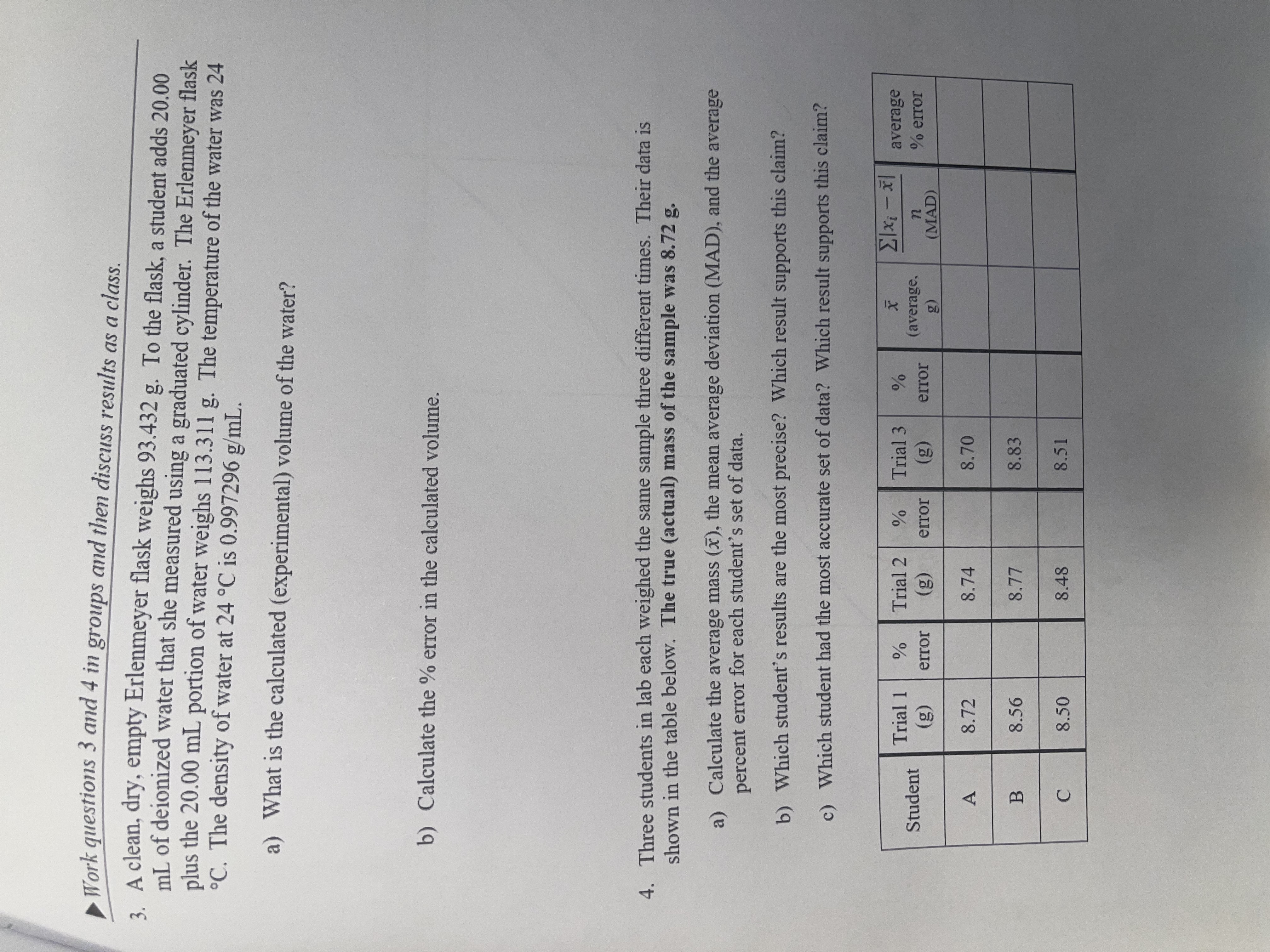
Work questions 3 and 4 in groups and then discuss results as a class. A clean, dry, empty Erlenmeyer flask weighs 9 3 . 4
Work questions and in groups and then discuss results as a class.
A clean, dry, empty Erlenmeyer flask weighs To the flask, a student adds
of deionized water that she measured using a graduated cylinder. The Erlenmeyer flask
plus the portion of water weighs The temperature of the water was
The density of water at is
a What is the calculated experimental volume of the water?
b Calculate the error in the calculated volume.
Three students in lab each weighed the same sample three different times. Their data is
shown in the table below. The true actual mass of the sample was
a Calculate the average mass the mean average deviation MAD and the average
percent error for each student's set of data.
b Which student's results are the most precise? Which result supports this claim?
c Which student had the most accurate set of data? Which result supports this claim?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


