Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Write a Lewis structure for ethyl alcohol, CH,CH,OH, showing all valence electrons. You do not have to consider stereochemistry. Explicitly draw all H atoms.

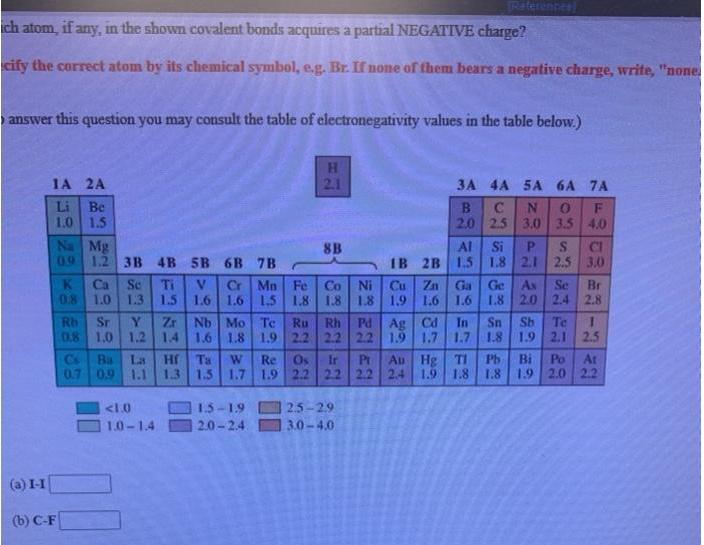


Write a Lewis structure for ethyl alcohol, CH,CH,OH, showing all valence electrons. You do not have to consider stereochemistry. Explicitly draw all H atoms. Include all valence lone pairs in your answer. Raterences ich atom, if any, in the shown covalent bonds acquires a partial NEGATIVE charge? cify the correct atom by its chemical symbol, e.g. Br. If none of them bears a negative charge, write, "none answer this question you may consult the table of electronegativity values in the table below.) 1A 2A 2.1 3A 4A 5A 6A 7A Be 1.0 1.5 Na Mg 09 12 3B 4B 5B 6B 7B Li 2.0 2.5 3,0 3.5 4.0 Al Si P. IB 2B 1.5 1.8 2.1 2,5 3.0 8B CI Cr Mn Fe 1.6 1.6 1.5 1.8 1.8 Co Ni Cu Zn 1.8 1.9 1.6 Ga Ge K Ca 0.8 Se Ti 1.3 Se Br 2.4 2.8 As 1.0 1.5 1.6 1.8 2.0 Zr 08 1.0 1.2 14 Nb Mo Te Pd Ag Cd 1.9 Rh Te Sb 1.9 2.1 2.5 Sr Rh In Sn 1.7 1.7 1.8 Ru 1.6 1.8 1.9 2.2 2.2 2.2 Au Hg TI Cs Bs 0.7 09 1.1 La Hr Ta 1.3 1.5 1.7 1.9 2.2 22 2.2 2.4 Ir Pr Pb B 1.8 Po At 1.9 2.0 2.2 W Re Os 1.9 1.8
Step by Step Solution
There are 3 Steps involved in it
Step: 1
1 Lewis ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


