Answered step by step
Verified Expert Solution
Question
1 Approved Answer
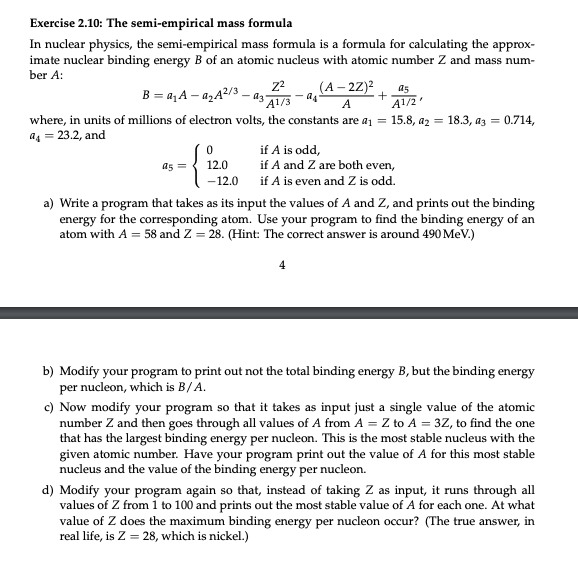
Write in Python please of the Jupyter Lab Notebook Exercise 2.10: The semi-empirical mass formula In nuclear physics, the semi-empirical mass formula is a formula
Write in Python please of the Jupyter Lab Notebook
Exercise 2.10: The semi-empirical mass formula In nuclear physics, the semi-empirical mass formula is a formula for calculating the approximate nuclear binding energy B of an atomic nucleus with atomic number Z and mass number A : B=a1Aa2A2/3a3A1/3Z2a4A(A2Z)2+A1/2a5, where, in units of millions of electron volts, the constants are a1=15.8,a2=18.3,a3=0.714, a4=23.2, and a5=012.012.0ifAisodd,ifAandZarebotheven,ifAisevenandZisodd. a) Write a program that takes as its input the values of A and Z, and prints out the binding energy for the corresponding atom. Use your program to find the binding energy of an atom with A=58 and Z=28. (Hint: The correct answer is around 490MeV.) 4 b) Modify your program to print out not the total binding energy B, but the binding energy per nucleon, which is B/A. c) Now modify your program so that it takes as input just a single value of the atomic number Z and then goes through all values of A from A=Z to A=3Z, to find the one that has the largest binding energy per nucleon. This is the most stable nucleus with the given atomic number. Have your program print out the value of A for this most stable nucleus and the value of the binding energy per nucleon. d) Modify your program again so that, instead of taking Z as input, it runs through all values of Z from 1 to 100 and prints out the most stable value of A for each one. At what value of Z does the maximum binding energy per nucleon occur? (The true answer, in real life, is Z=28, which is nickel.)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


