Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Wtfe Cmpd / MW Fe Cmpd MnO4 5* MMnO, * VM [2-2] When Completing The Data Sheet, Along With Sample (Example) Calculations On The Back,
Wtfe Cmpd / MW Fe Cmpd MnO4 5* MMnO, * VM [2-2] When Completing The Data Sheet, Along With Sample (Example) Calculations On The Back, Bear In Mind The Following Two Items. 1. Using Equation [2-2), And Given The Molecular Weight (MW, Or Molar Mass) Of Hydrated Ferrous Ammonium Sulfate As 392.14 G/Mol, Calculate The Molarity Of The Potassium Permanganate
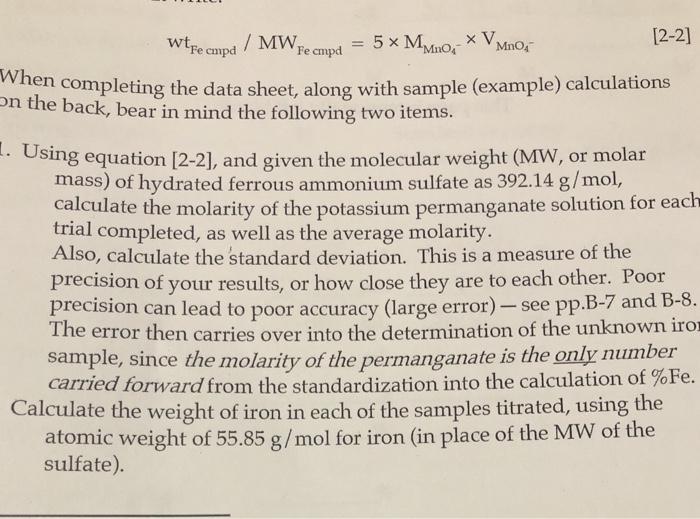
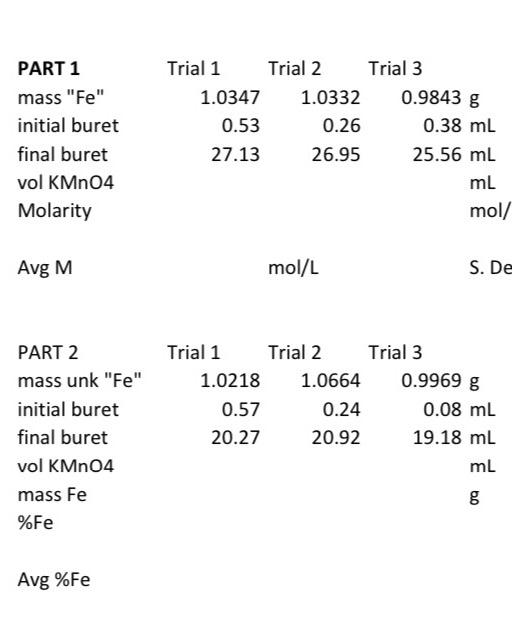
wtfe ampd /MW, = 5 x MMno V MnO Fe cmpd [2-2] When completing the data sheet, along with sample (example) calculations On the back, bear in mind the following two items. Using equation [2-2], and given the molecular weight (MW, or molar mass) of hydrated ferrous ammonium sulfate as 392.14 g/mol, calculate the molarity of the potassium permanganate solution for each trial completed, as well as the average molarity. Also, calculate the standard deviation. This is a measure of the precision of your results, or how close they are to each other. Poor precision can lead to poor accuracy (large error) - see pp.B-7 and B-8. The error then carries over into the determination of the unknown iron sample, since the molarity of the permanganate is the only number carried forward from the standardization into the calculation of %Fe. Calculate the weight of iron in each of the samples titrated, using the atomic weight of 55.85 g/mol for iron (in place of the MW of the sulfate).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


