Answered step by step
Verified Expert Solution
Question
1 Approved Answer
xylenes project. please draw out pfd and show work. Problem Statement: Xylenes are used as raw materials for the manufacturing of polyesters which are used
xylenes project. please draw out pfd and show work. 

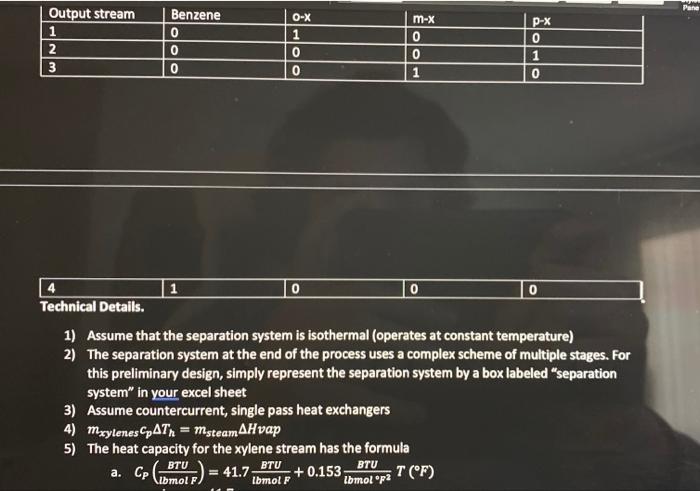


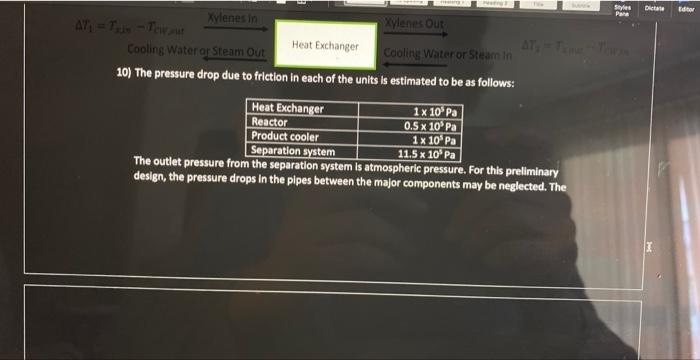


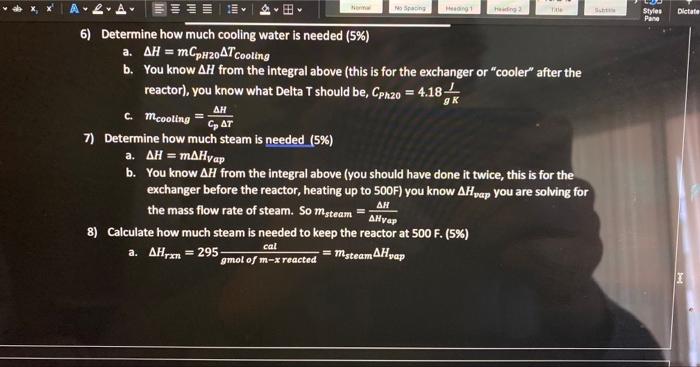
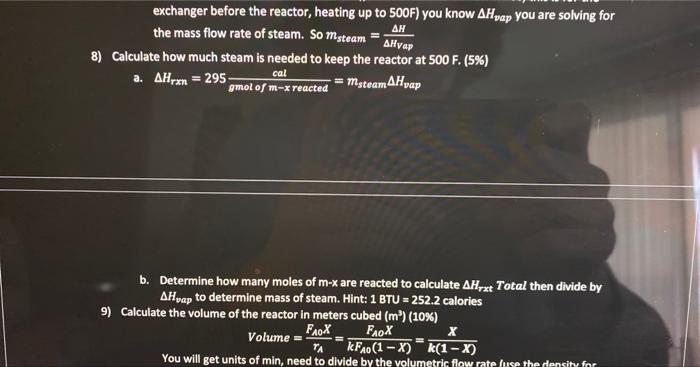

Problem Statement: Xylenes are used as raw materials for the manufacturing of polyesters which are used for textile fibers, photographic film, and soft-drink bottles. The process to be added to an existing facility involves the isomerization reactor where meta-xylenes (mx) is converted to ortho-xylenes (0x) and para-xylenes (p-x). Note that all three of these compounds are isomers (same elemental composition with different chemical structure). Therefore, they have the same molecular weight ( MW=106). For our purposes here we will assume that equal amounts of ox and px are produced so that the reaction proceeds as follows: 2mxrxnox+px This liquid phase reaction is assumed to be irreversible and is approximated by first-order kinetics with a rate constant of kr=0.133min1. The reaction is slightly endothermic (Hreact= 295gmolofmxreactedcal ) The simplified process is described below. A liquid feed stream will enter the process at 2atm and a temperature off 77F at a rate of 1.15 million kg/day. The mass fractions of that feed stream are: The feed stream goes through a distillation column where 99% (by mass) of the benzene is removed in the distillate (the top of the distillation column at 80F and 1atm ), the small amount of benzene and the entire xylene stream comes out in the bottoms at atmospheric pressure (1atm) and 283F. A centrifugal pump will be used to increase the pressure of the stream sufficiently to overcome the pressure drop due to friction in each of the components of the process (see the additional information given below). Following the pump, the stream will pass through a heat exchanger where the temperature will be increased from 283F to 500F using steam. The hot pressurized stream will then be fed into a well-mixed isothermal reactor (maintained with a steam jacket around the reactor) where 70% of the mx will be reacted to form 0x and px. The product stream from the reactor is cooled as much as possible with cooling water (100F) in another heat exchanger before entering the separation system. The separation system will yield four streams with the following mass fractions and which are all the same temperature. Technical Details. 1) Assume that the separation system is isothermal (operates at constant temperature) 2) The separation system at the end of the process uses a complex scheme of multiple stages. For this preliminary design, simply represent the separation system by a box labeled "separation system" in your excel sheet 3) Assume countercurrent, single pass heat exchangers 4) mxylenescpTh=msteam Hvap 5) The heat capacity for the xylene stream has the formula a. CP(lbmolFBTU)=41.7lbmolFBTU+0.153lbmolF2BTUT(F) 1) Assume that the separation system is isothermal (operates at constant temperature) 2) The separation system at the end of the process uses a complex scheme of multiple stages. For this preliminary design, simply represent the separation system by a box labeled "separation system" in your excel sheet 3) Assume countercurrent, single pass heat exchangers 4) mxylenescpTh=msteamH vap 5) The heat capacity for the xylene stream has the formula 1. a=41.7 ii. b=0.153 iii. Use Temperature in Fahrenheit not kelvin 6) The density of xy lenes is given by the equation A=49.9367;B=0.216174;C=619.996;D=0.23776(m3kg)=B1+(1cT)DA0.216174(1+(1619976T(K))0.3766 7) Saturated steam is available at 545F(1000psig) and Hvap=650LmBrU. When used for the heater and reactor, the amount of steam that is used is only that which is condensed, and the if. b=0.153 iii. Use Temperature in Fahrenheit not kelvin 6) The density of xylenes is given by the equation =B1+(1c)DAA=49.9367;B=0.216174;C=619.996;D=0.23776(m3kg)=0.216174(1+(16196)6(6))exs.49.9367 7) Saturated steam is available at 545F(1000psig) and Hvep=650Lmmru. When used for the heater and reactor, the amount of steam that is used is only that which is condensed, and the condensate leaves as a saturated liquid. 8) Cooling water is available at 90%F and has a maximum outlet temperature of 120%F. The heat capacity of water is 1sCcal or 4.18gCl or 1EFFBru 9) In selecting the temperature of the process stream leaving the cooler, observe the rule of them that the minimum temperature difference (either T1 or T2 ) for a heat exchanger is 10F, which means the Temp exciting the Xylene stream will be 100F 10) The pressure drop due to friction in each of the units is estimated to be as follows: The outlet pressure frolutue separation system is atmospheric pressure. For this preliminary design, the pressure drops in the plpes between the major components may be neglected. The The outlet pressure from the separation system is atmospheric pressure. For this preliminary design, the pressure drops in the plpes between the major components may be neglected. The pipes are all the same diameter and there are no significant changes in elevation The pump has an efficiency of 75%(=0.75) Assignment 1) Sketch the process on a blank sheet of paper (10\%) a. The purpose of this is for you to understand the process better and for you to be able to perform the mass balances. Please take a photo or scan this and upload it with your final assignment 2) Perform a mass balance on the process, one unit at 1) Sketch the process on a blank sheet of paper (10\%) a. The purpose of this is for you to understand the process better and for you to be able to perform the mass balances. Please take a photo or scan this and upload it with your final assignment 2) Perform a mass balance on the process, one unit at a time to determine the flows of each type of xylene and the benzene in the process (15\%) a. I would recommend that you do these by hand at first before you enter them into excel. 3) Put the mass balance in excel so that if one value changes the entire process automatically updates. Hint: the cells need to be linked and functions of one another (Part of 20% see note below) 4) Determine the enthalpy of heating up the xylene stream (before the reactor) and cooling down the xylene stream (after the reactor). Copy the enthalpy worksheet you made for HW4(15%) a. H=xxyleneCp(T)dT b. The first heat exchanger takes the stream from the distillation column at 283 to 500F c. The second heat exchanger (or the cooler) cools the hot stream from the reactor from 500F to 100F d. Will need to make two "enthalpy" sections on your workbook 5) Write an excel function for the density of xylene from 300K to 600K in a new sheet in the workbook. Create a graph where density is on the y axis and temperature is on the x axis a. Need to make sure you follow the rules of making a good graph. Include axis labels, tick marks, etc. b. I would make a separate column for Kelvin temperatures and Fahrenheit Temperatures 6) Determine how much cooling water is needed (5\%) a. H=mCpH20TCooling b. You know H from the integral above (this is for the exchanger or "cooler" after the reactor), you know what Delta T should be, CPh20=4.18gKJ c. mcooling=cpTH 7) Determine how much steam is needed (5\%) a. H=mHVap b. You know H from the integral above (you should have done it twice, this is for the exchanger before the reactor, heating up to 500F ) you know Hvap you are solving for the mass flow rate of steam. So msteam=HVapH 8) Calculate how much steam is needed to keep the reactor at 500F. (5\%) a. Hrxn=295gmolofmxreactedcal=msteamHvap exchanger before the reactor, heating up to 500F ) you know Hvap you are solving for the mass flow rate of steam. So msteam=HVapH 8) Calculate how much steam is needed to keep the reactor at 500F. (5\%) a. Hrn=295gmolofmxreactedcal=msteamHvap b. Determine how many moles of mx are reacted to calculate Hrxt Total then divide by Hvap to determine mass of steam. Hint: 1BTU=252.2 calories 9) Calculate the volume of the reactor in meters cubed (m3)(10%) Volume=rAFA0X=kFA0(1X)FAOX=k(1X)X b. Determine how many moles of mx are reacted to calculate Hrxt Total then divide by Hvap to determine mass of steam. Hint: 1BTU=252.2 calories 9) Calculate the volume of the reactor in meters cubed (m3)(10%) Volume=rAFA0X=kFA0(1X)FAOX=k(1X)X You will get units of min, need to divide by the volumetric flow rate (use the density for 500F(533K ) of the Xylene Stream). Volumetric flow rate = mass flow rate X density Volume=Volume=k(1X)sooXmxylreactor X=70% (Link this up to the conversion rate cell in your excel workbook, mass flow rate of the xylenes into the reactor) 10) Calculate the size of the pump that is needed. (5\%) a. Add up all the pressure drops listed in the table in the technical details section b. WP=Pm Use density in kg/m3 and mass flow rate in kg/s i. (remember 1kg=2.2lbs ) c. Units will come out to Watts (Joules/second) 










Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


