Question
Using the information below, calculate the cost of each of the energy producing reactions tested. CHEMICAL PRICE LIST Cost of HCI: $29.50/20 L bottle
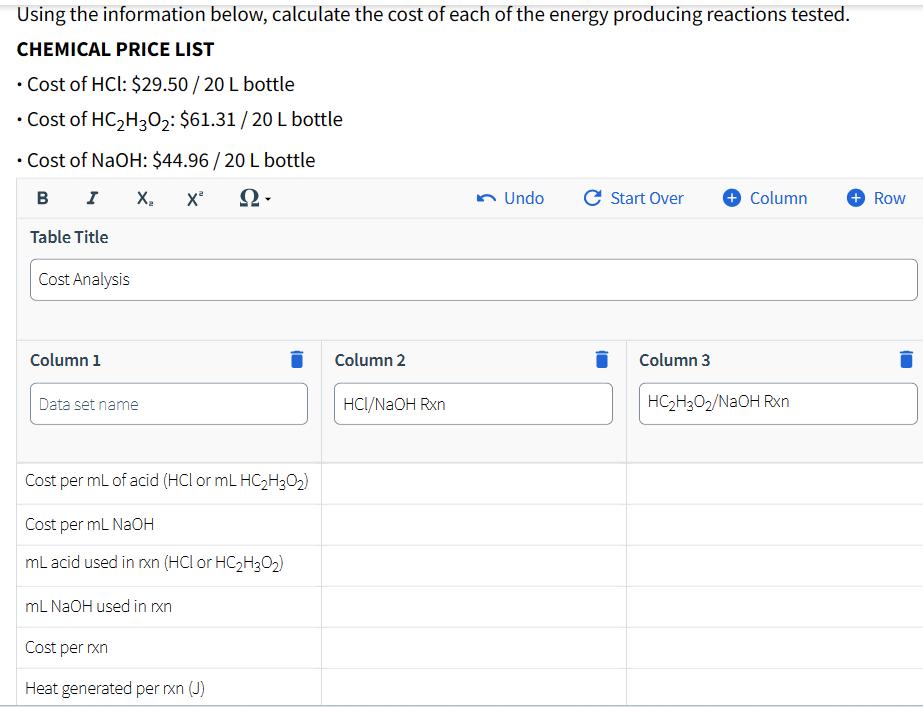
Using the information below, calculate the cost of each of the energy producing reactions tested. CHEMICAL PRICE LIST Cost of HCI: $29.50/20 L bottle Cost of HC2H3O2: $61.31/20 L bottle Cost of NaOH: $44.96/20 L bottle B I X x 2- Table Title Cost Analysis Column 1 Data set name Cost per mL of acid (HCl or mL HC2H3O2) Cost per mL NaOH mL acid used in rxn (HCl or HC2H3O2) mL NaOH used in rxn Cost per rxn Heat generated per rxn (J) Undo C Start Over + Column Row Column 2 Column 3 HCl/NaOH Rxn HC2H3O2/NaOH Rxn
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
To calculate the cost of each energyproducing reaction we will use the given information and apply t...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamental Managerial Accounting Concepts
Authors: Thomas Edmonds, Christopher Edmonds, Bor Yi Tsay, Philip Olds
8th edition
978-1259569197
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



