Answered step by step
Verified Expert Solution
Question
1 Approved Answer
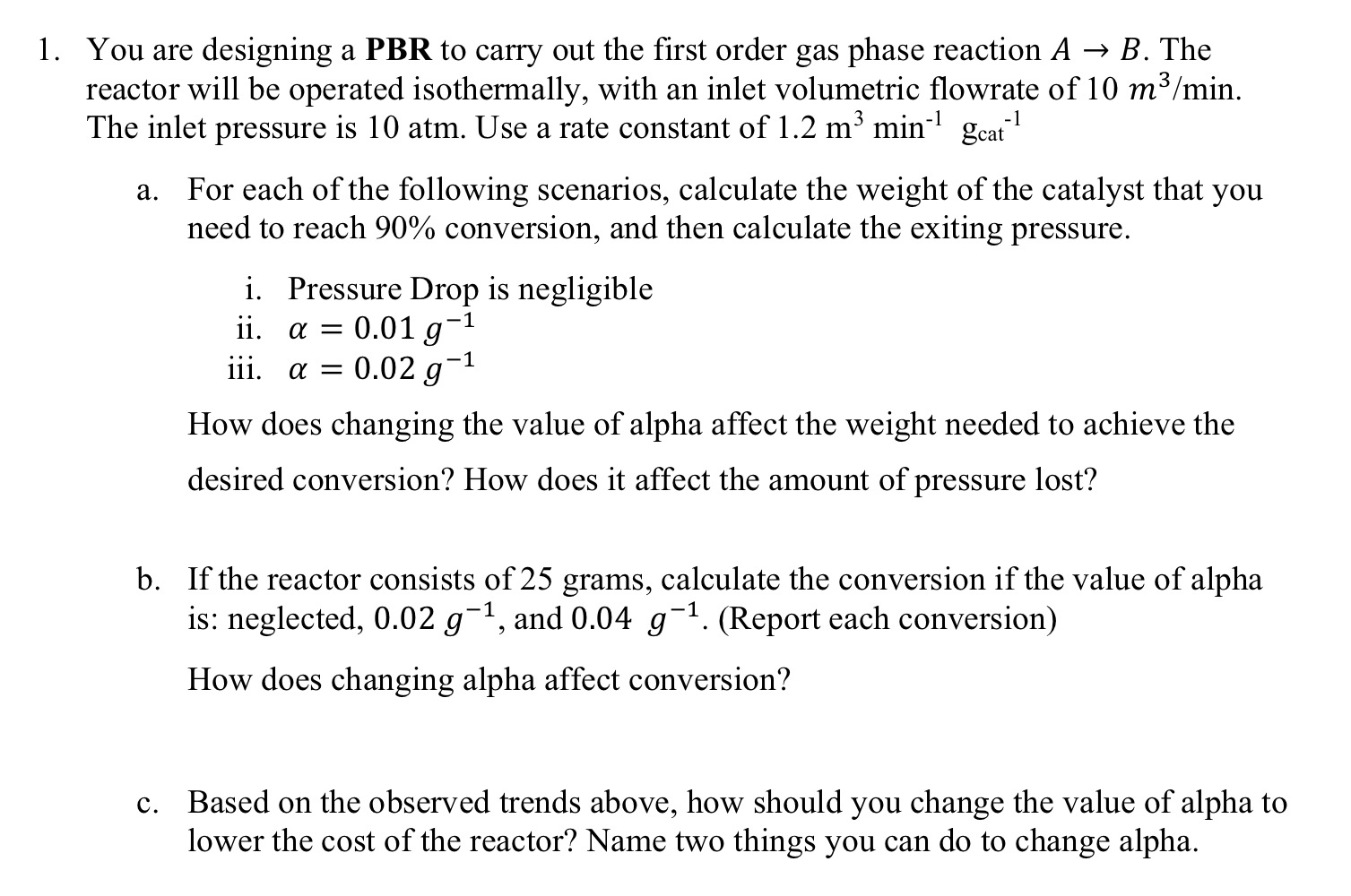
You are designing a PBR to carry out the first order gas phase reaction A B . The reactor will be operated isothermally, with an
You are designing a PBR to carry out the first order gas phase reaction The
reactor will be operated isothermally, with an inlet volumetric flowrate of
The inlet pressure is atm. Use a rate constant of
a For each of the following scenarios, calculate the weight of the catalyst that you
need to reach conversion, and then calculate the exiting pressure.
i Pressure Drop is negligible
ii
iii.
How does changing the value of alpha affect the weight needed to achieve the
desired conversion? How does it affect the amount of pressure lost?
b If the reactor consists of grams, calculate the conversion if the value of alpha
is: neglected, and Report each conversion
How does changing alpha affect conversion?
c Based on the observed trends above, how should you change the value of alpha to
lower the cost of the reactor? Name two things you can do to change alpha.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


