Question
Calculate the cell potential and the equilibrium constant for the following reaction at 298 K: Sn2+(aq) + Cu(s) >> Sn(s) + Cu+(aq) Hint: Carry
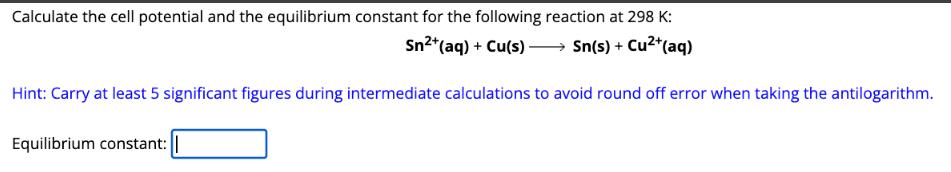
Calculate the cell potential and the equilibrium constant for the following reaction at 298 K: Sn2+(aq) + Cu(s) >> Sn(s) + Cu+(aq) Hint: Carry at least 5 significant figures during intermediate calculations to avoid round off error when taking the antilogarithm. Equilibrium constant: ||
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solution To calculate the cell potential and the equilibrium constant for the reaction Snaq Cus Sns ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Derivative Pricing
Authors: Ambrose Lo
1st Edition
0367734214, 978-0367734213
Students also viewed these Finance questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



