Answered step by step
Verified Expert Solution
Question
1 Approved Answer
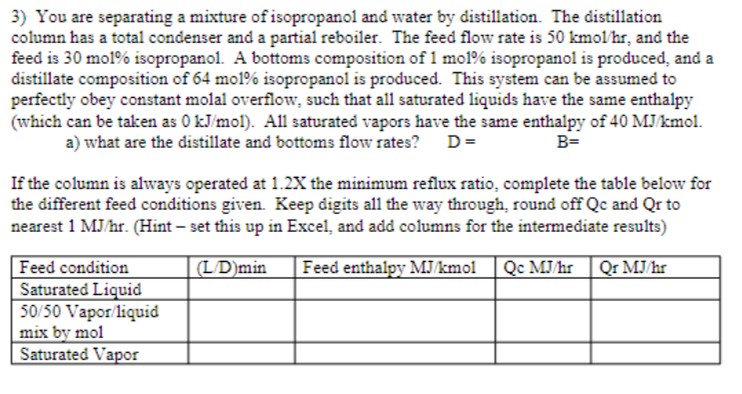
You are separating a mixture of isopropanol and water by distillation. The distillation column has a total condenser and a partial reboiler. The feed flow
You are separating a mixture of isopropanol and water by distillation. The distillation column has a total condenser and a partial reboiler. The feed flow rate is kmolhr and the feed is mol isopropanol. A bottoms composition of mol isopropanol is produced, and a
distillate composition of mol isopropanol is produced. This system can be assumed to perfectly obey constant molal overflow, such that all saturated liquids have the same enthalpy which can be taken as kJmol All saturated vapors have the same enthalpy of MJkmol
a what are the distillate and bottoms flow rates?
If the column is always operated at the minimum reflux ratio, complete the table below for the different feed conditions given. Keep digits all the way through, round off Qc and Qr to nearest MJhr Hint set this up in Excel, and add columns for the intermediate results
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


