Answered step by step
Verified Expert Solution
Question
1 Approved Answer
YOU CAN JUST SOLVE QUESTION #1, I HAVE SOLVED THE REST BUT I POSTED IT FOR YOU TO MAKE SURE THEY ARE RIGHT, SO PLZ
YOU CAN JUST SOLVE QUESTION #1, I HAVE SOLVED THE REST BUT I POSTED IT FOR YOU TO MAKE SURE THEY ARE RIGHT, SO PLZ LEAVE AN OPINION IN EACH AND TELL ME IF THEY ARE RIGH OR WRONG, THANKS!
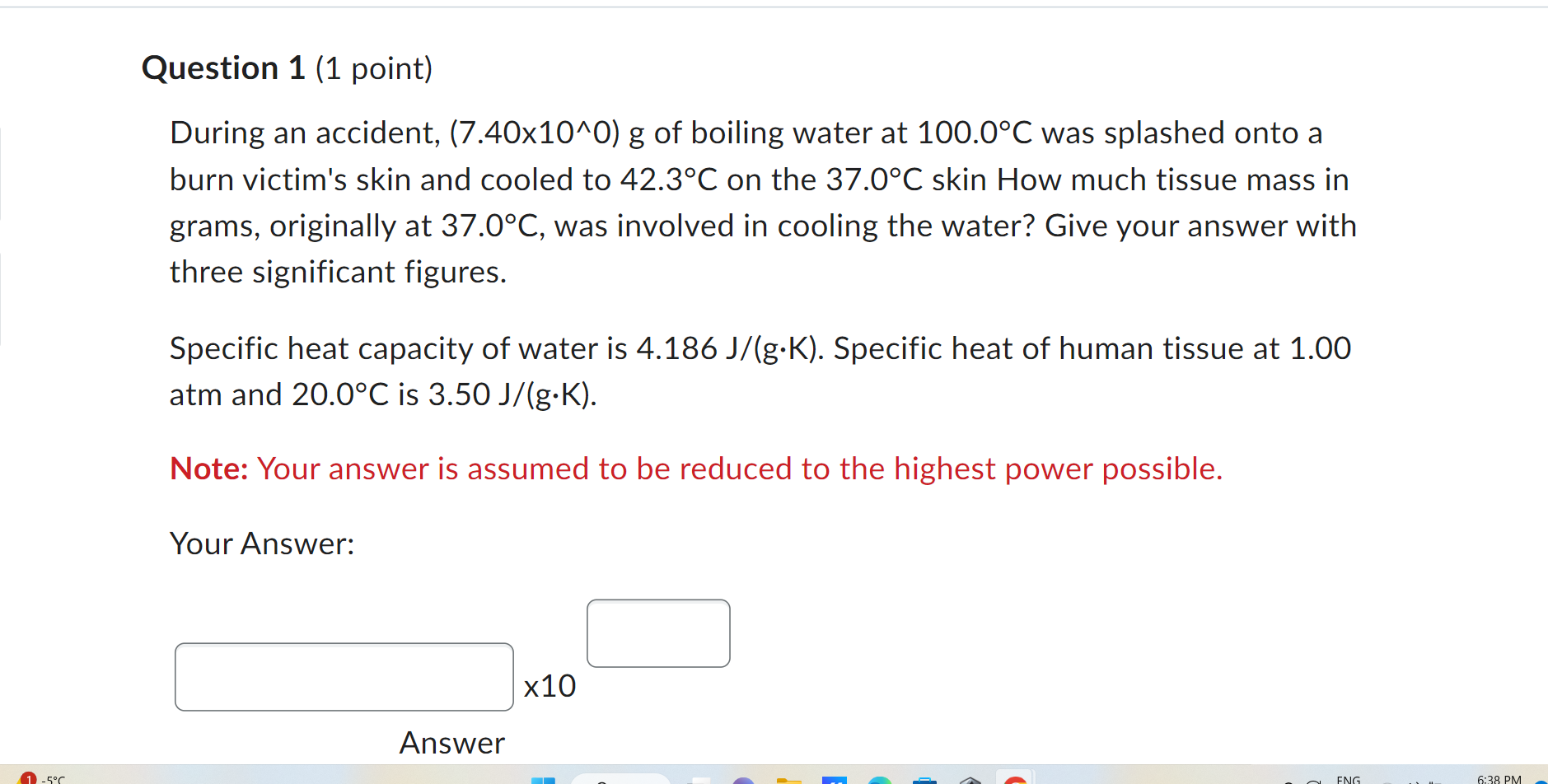 Question 1 (1 point) During an accident, (7.40x10^0) g of boiling water at 100.0 C was splashed onto a burn victim's skin and cooled to 42.3C on the 37.0 C skin How much tissue mass in grams, originally at 37.0 C, was involved in cooling the water? Give your answer with three significant figures. Specific heat capacity of water is 4.186 J/(g.K). Specific heat of human tissue at 1.00 atm and 20.0 C is 3.50 J/(g.K). Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10
Question 1 (1 point) During an accident, (7.40x10^0) g of boiling water at 100.0 C was splashed onto a burn victim's skin and cooled to 42.3C on the 37.0 C skin How much tissue mass in grams, originally at 37.0 C, was involved in cooling the water? Give your answer with three significant figures. Specific heat capacity of water is 4.186 J/(g.K). Specific heat of human tissue at 1.00 atm and 20.0 C is 3.50 J/(g.K). Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


