Answered step by step
Verified Expert Solution
Question
1 Approved Answer
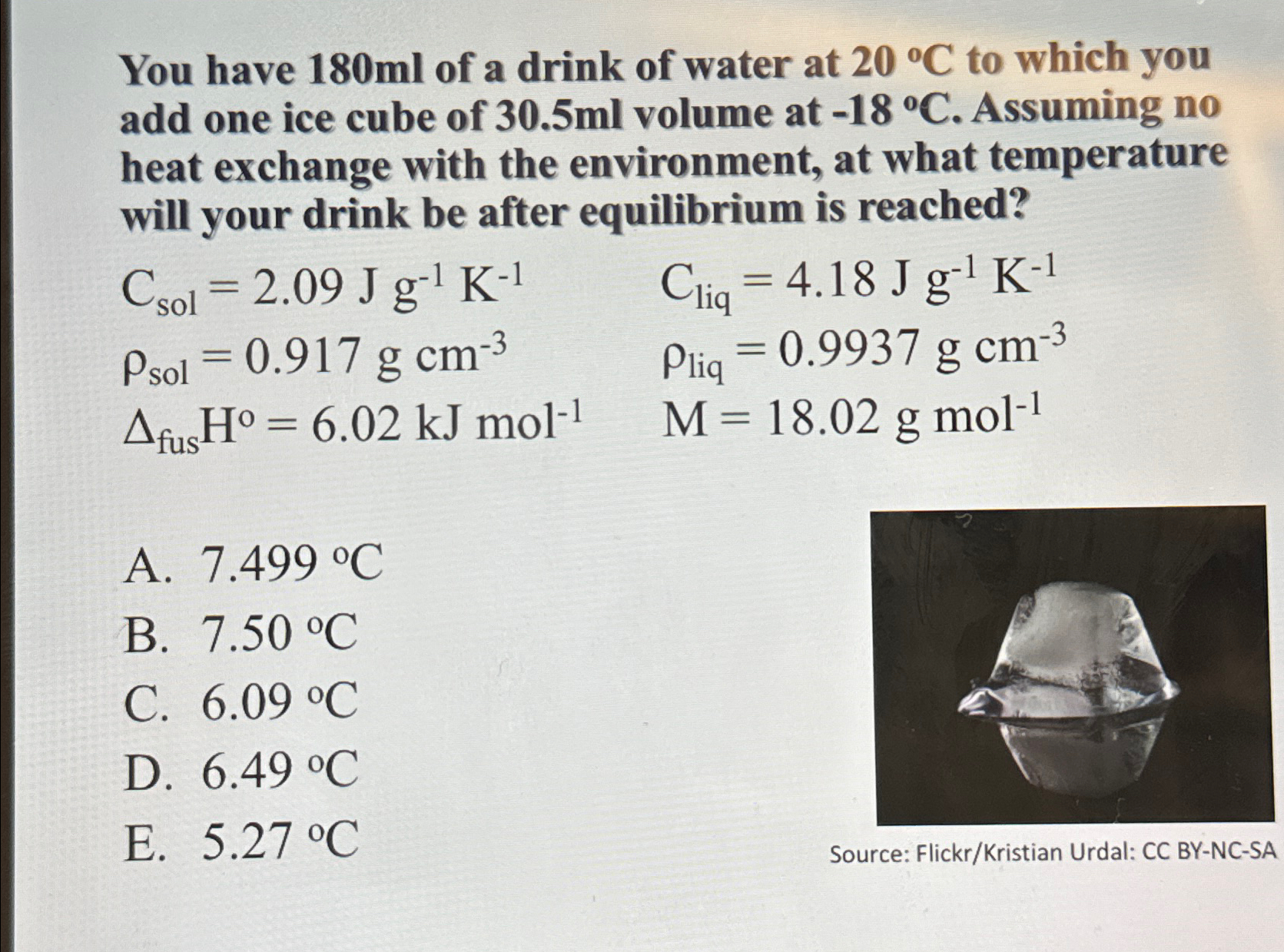
You have 180ml of a drink of water at 20deg C to which you add one ice cube of 30.5ml volume at -18deg C .
You have
180mlof a drink of water at
20\\\\deg Cto which you add one ice cube of
30.5mlvolume at
-18\\\\deg C. Assuming no heat exchange with the environment, at what temperature will your drink be after equilibrium is reached?\
C_(sol )=2.09Jg^(-1)K^(-1),C_(liq )=4.18Jg^(-1)K^(-1)\ \\\ ho _(sol )=0.917gcm^(-3),\\\ ho _(liq )=0.9937gcm^(-3)\ \\\\Delta _(fus )H^(0)=6.02kJmol^(-1),M=18.02gmol^(-1)\ A.
7.499\\\\deg C\ B.
7.50\\\\deg C\ C.
6.09\\\\deg C\ D.
6.49\\\\deg C\ E.
5.27\\\\deg C\ Source: Flickr/Kristian Urdal: CC BY-NC-SA
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


