Question
Computational studies have determined the effective nuclear charge, Zeff, for an electron in a particular subshell to a high degree of certainty. The table
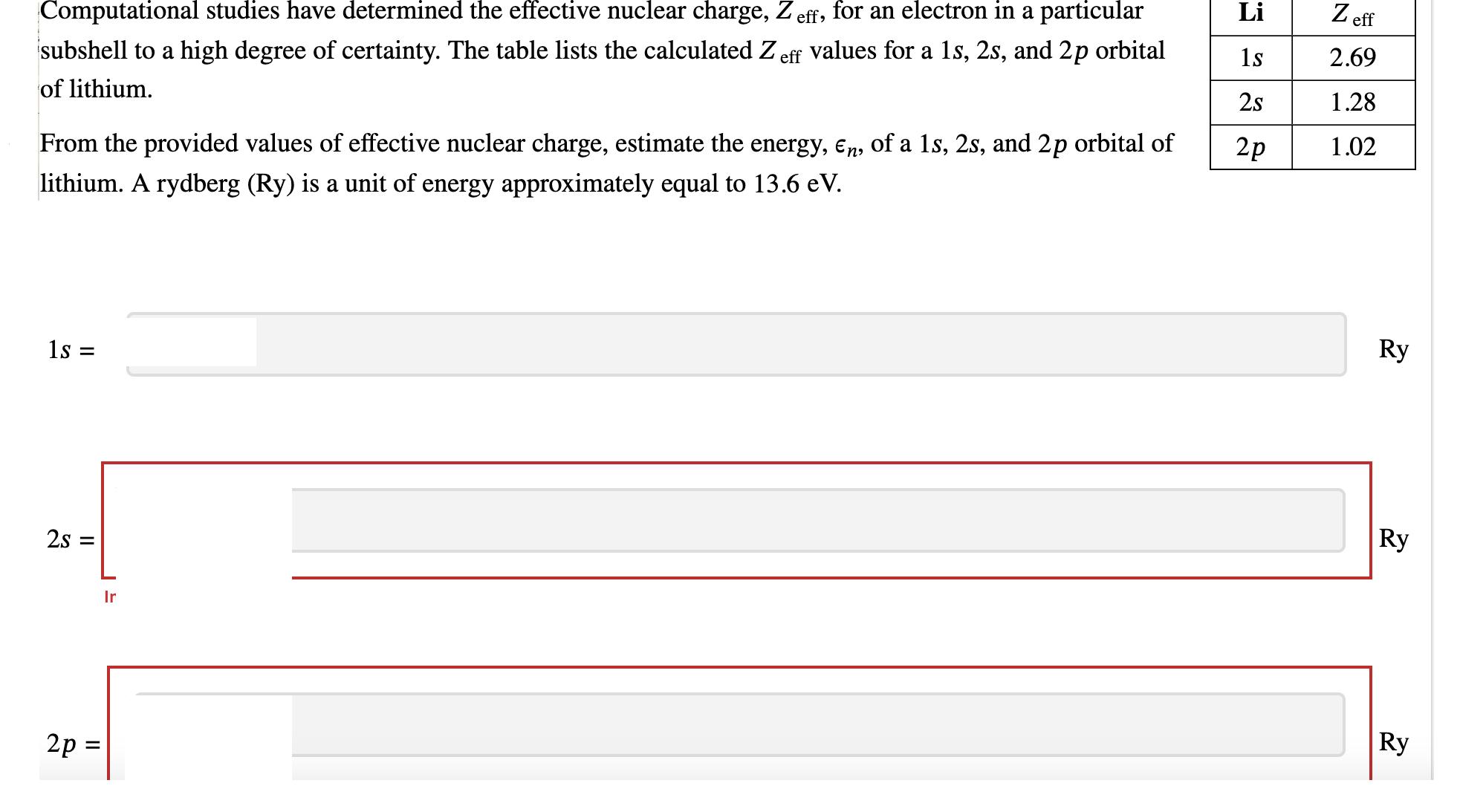
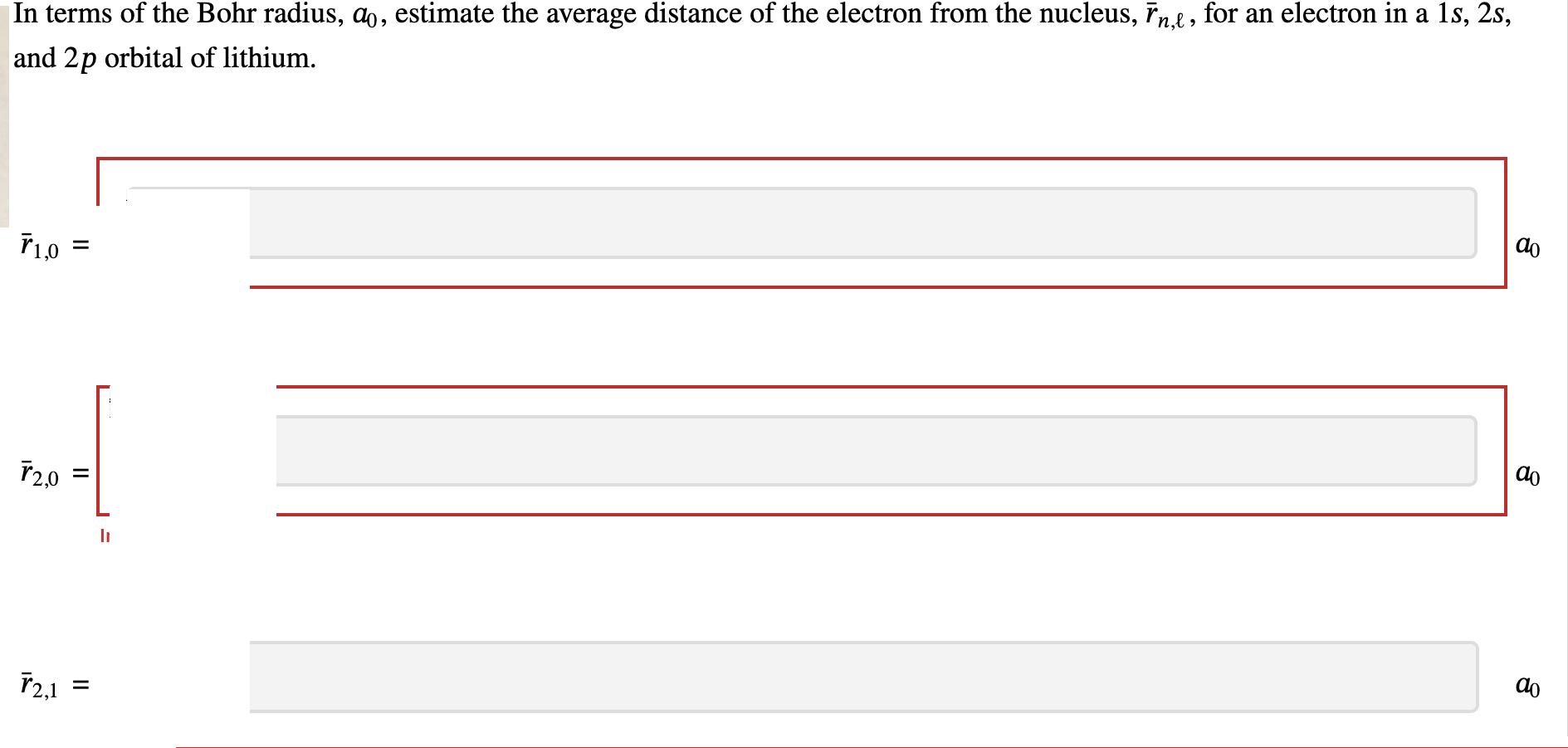
Computational studies have determined the effective nuclear charge, Zeff, for an electron in a particular subshell to a high degree of certainty. The table lists the calculated Z eff values for a 1s, 2s, and 2p orbital of lithium. From the provided values of effective nuclear charge, estimate the energy, n, of a 1s, 2s, and 2p orbital of lithium. A rydberg (Ry) is a unit of energy approximately equal to 13.6 eV. 1s = 2s = 2p= Ir Li 1s 2s 2p Zeff 2.69 1.28 1.02 Ry Ry Ry In terms of the Bohr radius, a, estimate the average distance of the electron from the nucleus, n,e, for an electron in a 1s, 2s, and 2p orbital of lithium. T1,0 = T2,0 T2,1 = = h do do do
Step by Step Solution
3.41 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus With Applications
Authors: Margaret L. Lial
12th Edition
978-0135871348, 0135871344
Students also viewed these Marketing questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



