Question
1. Write the net ionic equation for the following 2. When setting up their experiment a student discovered that the solution in their flask turned
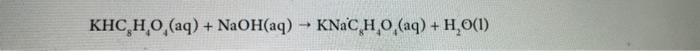
2. When setting up their experiment a student discovered that the solution in their flask turned a bright pink upon adding two drops of the indicator even before beginning the titration. What do you suspect was the mistake that the student made?
KHC HO (aq) + NaOH(aq) KNaC HO (aq) + HO(1)
Step by Step Solution
3.52 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
The Practice Of Statistics
Authors: Daren S. Starnes, Josh Tabor
6th Edition
978-1319113339
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



