Answered step by step
Verified Expert Solution
Question
1 Approved Answer
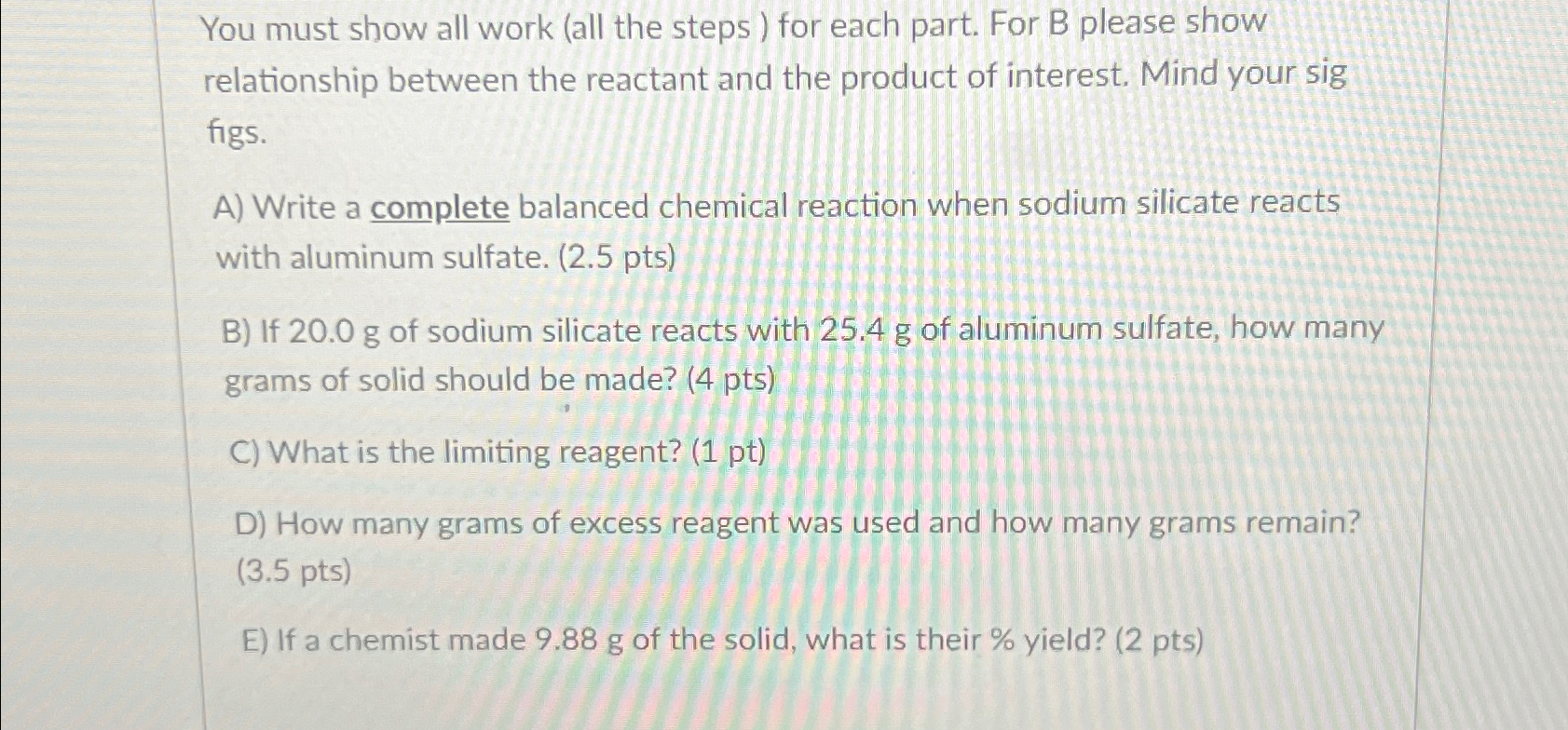
You must show all work ( all the steps ) for each part. For B please show relationship between the reactant and the product of
You must show all work all the steps for each part. For B please show relationship between the reactant and the product of interest. Mind your sig figs.
A Write a complete balanced chemical reaction when sodium silicate reacts with aluminum sulfate.
B If of sodium silicate reacts with of aluminum sulfate, how many grams of solid should be made? pts
What is the limiting reagent? pt
D How many grams of excess reagent was used and how many grams remain?
pts
E If a chemist made of the solid, what is their yield? pts
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


