Answered step by step
Verified Expert Solution
Question
1 Approved Answer
You put 1.08 liter (1.08 kg) of room-temperature (20C) water (Cwater = 4190 J/kg/K ) into an electric pot that is turned on and
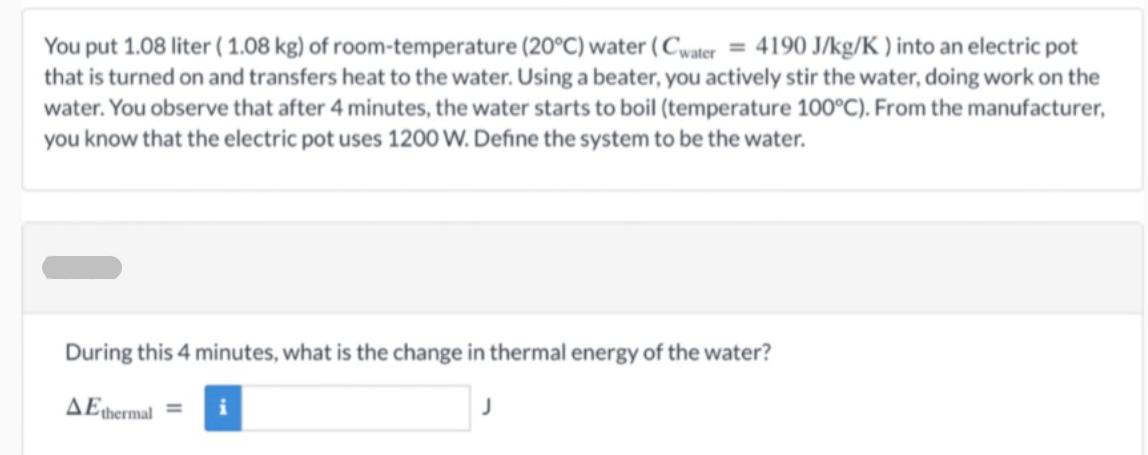
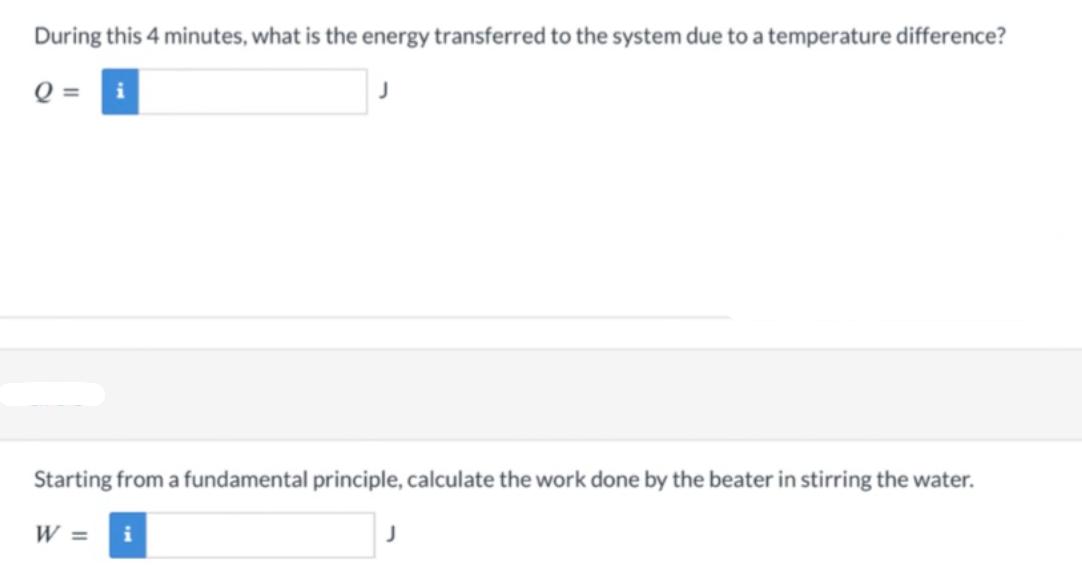
You put 1.08 liter (1.08 kg) of room-temperature (20C) water (Cwater = 4190 J/kg/K ) into an electric pot that is turned on and transfers heat to the water. Using a beater, you actively stir the water, doing work on the water. You observe that after 4 minutes, the water starts to boil (temperature 100C). From the manufacturer, you know that the electric pot uses 1200 W. Define the system to be the water. During this 4 minutes, what is the change in thermal energy of the water? AE thermal i During this 4 minutes, what is the energy transferred to the system due to a temperature difference? Q = i Starting from a fundamental principle, calculate the work done by the beater in stirring the water. W = i
Step by Step Solution
★★★★★
3.56 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION Calculating the thermal energy within the first 4 minutes Mass of the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


