Answered step by step
Verified Expert Solution
Question
1 Approved Answer
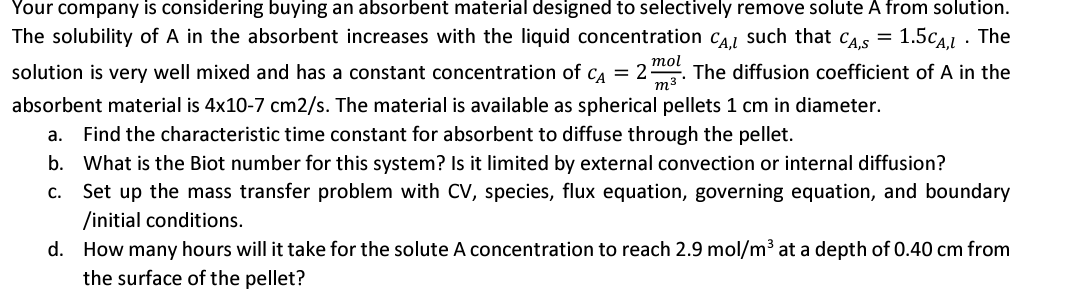
Your company is considering buying an absorbent material designed to selectively remove solute A from solution. The solubility of A in the absorbent increases with
Your company is considering buying an absorbent material designed to selectively remove solute A from solution.
The solubility of in the absorbent increases with the liquid concentration such that The
solution is very well mixed and has a constant concentration of The diffusion coefficient of in the
absorbent material is The material is available as spherical pellets in diameter.
a Find the characteristic time constant for absorbent to diffuse through the pellet.
b What is the Biot number for this system? Is it limited by external convection or internal diffusion?
c Set up the mass transfer problem with CV species, flux equation, governing equation, and boundary
initial conditions.
d How many hours will it take for the solute A concentration to reach at a depth of from
the surface of the pellet?Your company is considering buying an absorbent material designed to selectively remove solute A from solution. The solubility of A in the absorbent increases with the liquid concentration such that The solution is very well mixed and has a constant concentration of The diffusion coefficient of A in the absorbent material is x cms The material is available as spherical pellets cm in diameter. a Find the characteristic time constant for absorbent to diffuse through the pellet. b What is the Biot number for this system? Is it limited by external convection or internal diffusion? c Set up the mass transfer problem with CV species, flux equation, governing equation, and boundary initial conditions. d How many hours will it take for the solute A concentration to reach molm at a depth of cm from the surface of the pellet?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


