Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Instruction: In the table below identify the conjugate bases that are also weak acids. Acid name Strong acid hydroiodic acid perchloric acid hydrobromic acid
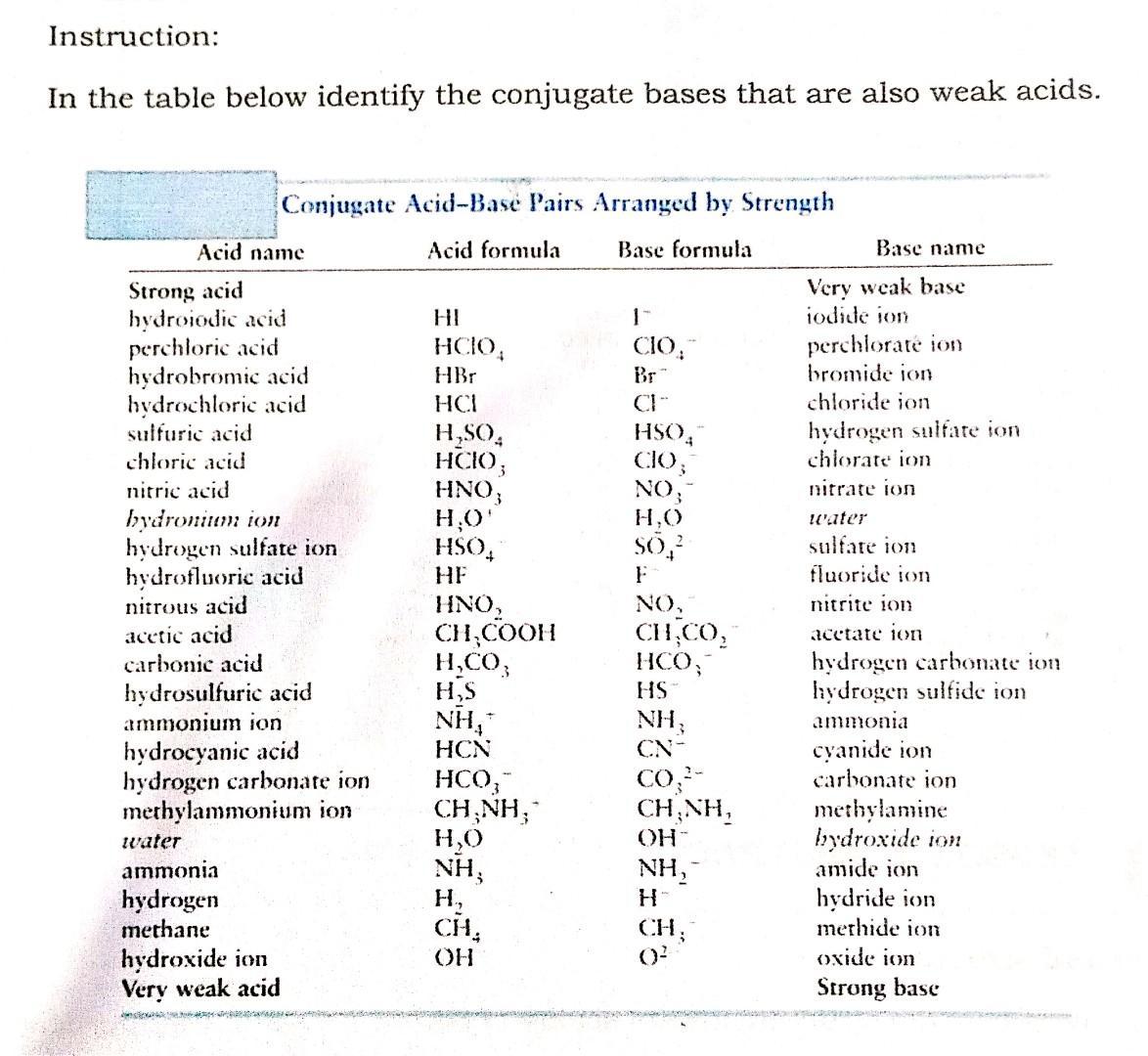
Instruction: In the table below identify the conjugate bases that are also weak acids. Acid name Strong acid hydroiodic acid perchloric acid hydrobromic acid hydrochloric acid sulfuric acid chloric acid nitric acid Conjugate Acid-Base Pairs Arranged by Strength Base formula Acid formula hydronium ion hydrogen sulfate ion hydrofluoric acid nitrous acid acetic acid carbonic acid hydrosulfuric acid ammonium ion hydrocyanic acid hydrogen carbonate ion methylammonium ion water ammonia hydrogen methane hydroxide ion Very weak acid HI HCIO, HBr HCI HSO4 HCIO; HNO, HO' HSO, HF HNO CHCOOH HCO3 H,S NH* HCN HCO, CHNH, HO NH H CH OH 1- 84+1829.10 CHNH, OH- NH H CH, Base name Very weak base iodide ion perchlorate ion bromide ion chloride ion hydrogen sulfate ion chlorate ion nitrate ion water sulfate ion fluoride ion nitrite ion acetate ion hydrogen carbonate ion hydrogen sulfide ion ammonia cyanide ion carbonate ion methylamine hydroxide ion amide ion hydride ion methide ion oxide ion Strong base
Step by Step Solution
★★★★★
3.40 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1
nsoy conjugate bese MCO MS Ning HS hydroge...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


