Question
Your science team has discovered some elemental metals on the planet. After testing each element, you have obtained the information given in the data chart
Your science team has discovered some elemental metals on the planet. After testing each element, you have obtained the information given in the data chart below. You have not yet been able to obtain the atomic masses. In addition, these are preliminary tests, so the degree of accuracy is not high. However, you now have enough information to analyse the periodic trends of these new elements.
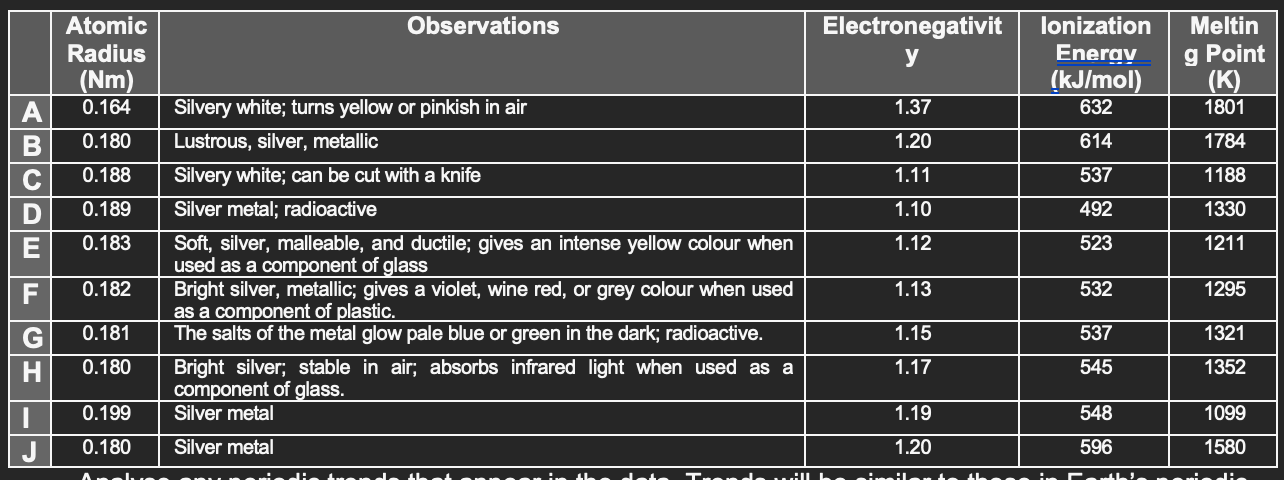
Analyse any periodic trends that appear in the data. Trends will be similar to those in Earths periodic table. (HINT: part of the list belongs to a group, and the other part belongs to a period)
- Explain the periodic trends (atomic radius, electronegativity, ionization energy, melting point) seen in the chart, as you go from A-J.
- How does your explanation correlate with Earths periodic table?
- Based on your answers from a & b, what elements are part of a group and which elements are part of a period.
- Which data does not seem to fit the trend? Can you suggest a possible explanation?
- One of the geologists on your team finds some fossilized wood on the planet. She attempts to find the age of the wood using carbon dating. In carbon dating, the relative amounts of carbon isotopes are compared. Since carbon isotopes decay at a regular rate, this information can be used to find the age of carbon compounds. However, the isotopic ratios of carbon on this planet are 80.2% 12C, 19.3% 13C, and 0.5% 14C. What is the average molar mass of carbon on the unknown planet?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


