Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Your system contains 100 g of a 50% methanol 50% water solution. a. What phase is the mixture at room temperature and pressure? b. What
Your system contains 100 g of a 50% methanol 50% water solution.
a. What phase is the mixture at room temperature and pressure? b. What is the boiling temperature of your solution at 1 atm? c. What is the composition of the liquid and vapor phases in equilibrium with each other at the temperature in part b? d. Describe the process you would use to increase the concentration of water in your system. Justify each step. e. What is the highest purity that you can achieve in terms of mol fraction of water with the distillation process?
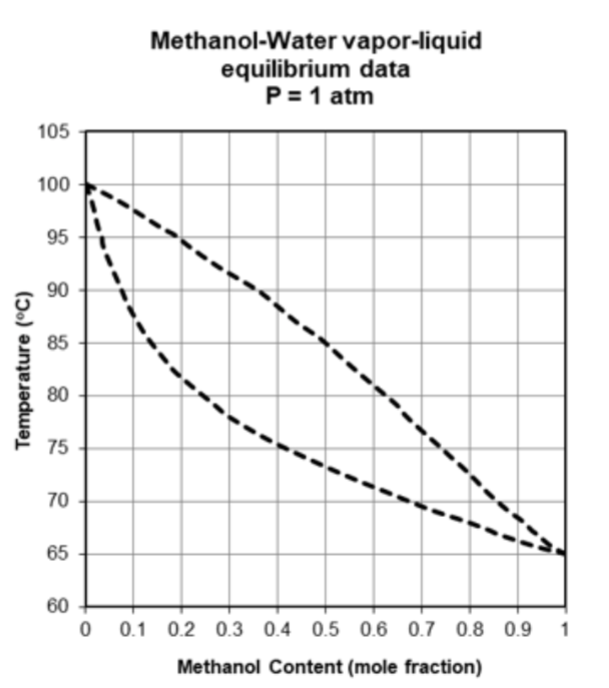
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


