Question
The boiling point of water, HO, is 100.000 C at 1 atmosphere. Ky(water) = 0.512 C/m In a laboratory experiment, students synthesized a new
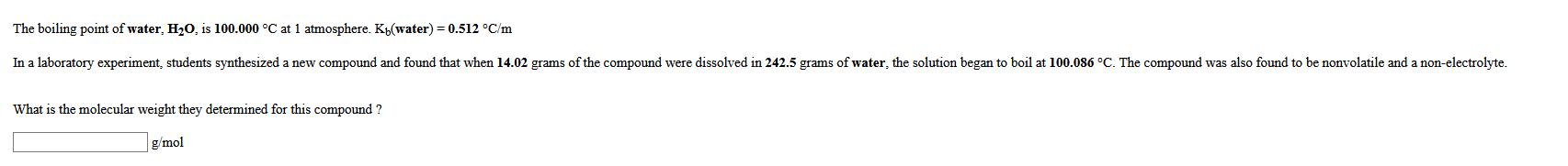
The boiling point of water, HO, is 100.000 C at 1 atmosphere. Ky(water) = 0.512 C/m In a laboratory experiment, students synthesized a new compound and found that when 14.02 grams of the compound were dissolved in 242.5 grams of water, the solution began to boil at 100.086 C. The compound was also found to be nonvolatile and a non-electrolyte. What is the molecular weight they determined for this compound? g/mol
Step by Step Solution
3.47 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Given Tbl 1000C Kb water 0512cm mass of Solute 1346g mass of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Intermediate Accounting
Authors: kieso, weygandt and warfield.
IFRS Edition
978-1118443965, 1118800532, 9781118800539, 978-0470873991
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



