Determine the work done by the gas in going from A to B in the thermodynamic cycle
Question:
Determine the work done by the gas in going from A to B in the thermodynamic cycle shown in Fig. 20-2. Repeat for portion CA. Give answers to one significant figure.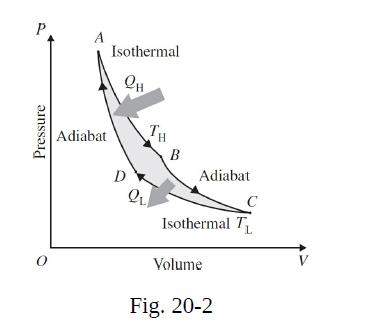
Transcribed Image Text:
P Pressure Adiabat Isothermal QH D TH QL B Adiabat Isothermal T L Volume C Fig. 20-2 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
04...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physics questions
-
Oil marketing companies have decided to perform an analysis on crude oil prices for n number of days. The aim is to find the number of consecutive days preceding the present-day [inclusive] when the...
-
One handred moles of a monatomic gas is com-pressed as shown on the pV diagram in Fig. 12.23. (a) Is the work done by the gas (1) positive, (2) zero, or (3) negative? Why? (b) What is the work done...
-
A monatomic ideal gas expands from point A to point B along the path shown in the drawing. (a) Determine the work done by the gas. (b) The temperature of the gas at point A is 185 K. What is its...
-
Provide an detailed overview on the topic indirect pay/benefits. Use the information given: Indirect Pay: any type of employer-provided reward (or "benefit") that serves an employee need but is not...
-
Van Buren Resources, Inc., is considering borrowing $100,000 for 182 days from its bank. Van Buren will pay $6,000 of interest at maturity, and it will repay the $100,000 of principal at maturity. a....
-
Hewlett-Packard (HP) is a leading direct marketer of computers and peripherals. The company made a profit of $7,074 million on sales of more than $127 billion in the year ended October 31, 2011. 1....
-
If a bond issue is to be sold at par, how will its coupon rate be determined? AppendixLO1
-
The following trial balance was extracted from the books of Old NV on 31 December 20X1. Note of information not taken into the trial balance data: (a) Provide for: (i) An audit fee of 38,000. (ii)...
-
On January 1, there were two jobs in process at the Bondview Company. Job No. J-1 J-2 Direct Materials $90 $20 Direct Labor 70 40 Materials Inventory at January 1 totaled $460. Materials purchased...
-
Find the net work output per cycle for the thermodynamic cycle in Fig. 20-6. Give your answer to two significant figures. P (atm) 6 4 2 0 D 5 Fig. 20-6 B 10 V (liters)
-
Water is boiled at 100 C and 1.0 atm. Under these conditions, 1.0 g of water occupies 1.0 cm 3 , 1.0 g of steam occupies 1670 cm 3 , and L = 540 cal/g. Find (a) The external work done when 1.0 g of...
-
Consider the same bill of material and information in Problem #8 but assume that two of component B are needed for each A, and that the gross requirement for A is 220 units. Compute the net...
-
Audio Partners needs to invest in the next level of technology in order to be competitive. The company is exploring the purchase of a new piece of equipment that will cost $1,500,000, at an expected...
-
Choose a real company of their choosing and will focus on ways to help increase the company's digital consumer engagements. For example, how can the company better drive increased revenue, sales,...
-
Four morally and ethically relevant principles have been examined regarding scarcity and include: Treating people with consistency through the use of a lottery or first-come first-served basis...
-
Critically evaluate the interplay between feedback skills, communication, listening, and assertiveness skills. How do these skills complement each other in the context of providing effective...
-
Generally, what was Starbucks intended strategy (first 2 minutes of video)? What is an emergent strategy mentioned in the video ( what changes made by Howard Schultz)? What were some (at least 2)...
-
Table 5.8 contains the continuously compounded forward rates ((0, T - (, T), where ( = 0.25. The first entry is the current spot rate, as for T = 0.25 we have ((0, 0, 0.25) = r(0,0.25). Compute the...
-
Sportique Boutique reported the following financial data for 2012 and 2011. Instructions(a) Calculate the current ratio for Sportique Boutique for 2012 and 2011.(b) Suppose that at the end of 2012,...
-
A circle is divided into 21 equal parts. A pointer is spun until it stops on one of the parts, which are numbered from 1 through 21. Describe the sample space and, assuming equally likely outcomes,...
-
The double-sided power spectral density of noise n (t) is shown in Figure 7.17. If n (t) = n c (t) cos(2Ïf 0 t + θ) - n s (t) sin(2Ïf 0 t + θ), find and plot S nc...
-
Noise n (t) has the power spectral density shown in Figure 7.16. We write n(t) = n c (t) cos (2Ïf 0 t + θ) - n s (t) sin(2Ïf 0 t + θ) Make plots of the power...
-
For journal entries 1 through 6 identify the explanation that most closely describes it. A. To record this period's depreciation expense. B. To record accrued salaries expense. C. To record this...
-
Julie Anderson is a project manager and her wage is 74,200. Julie's father passed away on April 14. She inherited her fathers cottage in Montana and 80,000 cash. She used the 80,000 to invest in an...
-
Complete the amortisation schedule POPCORN Corporation acquired an 80% interest in the outstanding stock of SALT Corporation for $520,000 on 1/1/X1. At this time, the stockholders' equity of SALT...

Study smarter with the SolutionInn App


